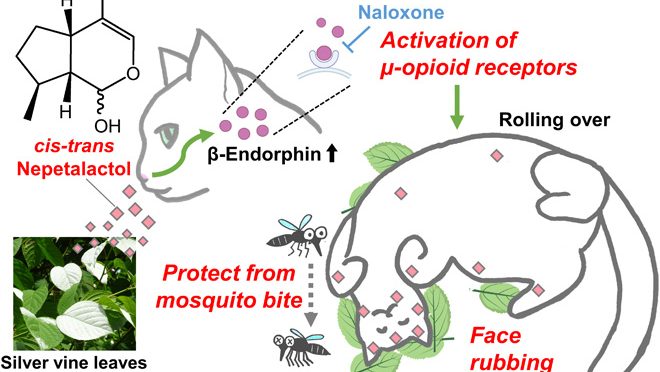
Domestic cats and other felids rub their faces and heads against catnip (Nepeta cataria) and silver vine (Actinidia polygama) and roll on the ground as a characteristic response. While this response is well known, its biological function and underlying mechanism remain undetermined. Here, we uncover the neurophysiological mechanism and functional outcome of this feline response. We found that the iridoid nepetalactol is the major component of silver vine that elicits this potent response in cats and other felids. Nepetalactol increased plasma β-endorphin levels in cats, while pharmacological inhibition of μ-opioid receptors suppressed the classic rubbing response. Rubbing behavior transfers nepetalactol onto the faces and heads of respondents where it repels the mosquito, Aedes albopictus. Thus, self-anointing behavior helps to protect cats against mosquito bites. The characteristic response of cats to nepetalactol via the μ-opioid system provides an important example of chemical pest defense using plant metabolites in nonhuman mammals.
INTRODUCTION
While learned behaviors allow animals to adapt flexibly to complex and changing environments, some species-specific behaviors are expressed reliably with no requirement for previous exposure or learning (1). This type of fixed behavior in animals is often elicited by chemical signals in the secretions of conspecifics (pheromones) or chemical cues from predators or prey (kairomones), where a reliably evoked behavioral response is important for survival. In addition, some plant odorants can also elicit characteristic responses in animals. A well-known example of plant-induced behavior in mammals is observed in domestic cats (Felis silvestris catus) and other felids such as lions (Panthera leo) and bobcats (Lynx rufus) (2–4). When felids sniff specific plants such as catnip (Nepeta cataria) and silver vine (Actinidia polygama), they exhibit a typical behavioral response that comprises licking and chewing the plants, face and head rubbing against the plants, and rolling over on the ground (2, 4, 5). This catnip and silver vine response usually lasts 5 to 15 min, followed by a period of one or more hours when they are nonresponsive (6). Because cats demonstrate an intoxicated response that does not have any pathophysiological effects (7), dried leaves of these plants are used commercially in toys for domestic cats worldwide.
The first reports of the feline behavioral response to silver vine and catnip were described by a Japanese botanist in 1704 (8) and by a British botanist in 1759 (9), respectively. The behavioral response to silver vine has been captured in Japanese culture: An Ukiyo-e (a type of traditional painting) drawn in 1859 depicts a folk story concerning a battle between cats and mice, wherein mice use silver vine as a weapon to intoxicate cats (10). While silver vine is endemic to Japan and China, its potent effects on cat behavior came to global recognition following its import from China to the United States (11). Bioactive iridoid compounds in catnip (nepetalactone) and silver vine (isoiridomyrmecin, iridomyrmecin, isodihydronepetalactone, and dihydronepetalactone) induce the same characteristic response (12–15). Cats perceive these secondary plant metabolites through the main olfactory system (6), while oral administration of nepetalactone induces no response (16). The strength of behavioral response increases with cat maturity (17), but there is no sexual dimorphism in response among adults. However, despite widespread recognition of this characteristic behavioral response to specific plants by feline carnivores, its functional outcome is not yet understood.
This study aimed to uncover the neurophysiological mechanism and biological function of the silver vine response in domestic cats. To establish a reliable and reproducible behavioral assay for precise control of stimulus presentation, we first purified potent bioactive compounds from silver vine leaves and identified nepetalactol, which had been missed in previous studies (13–15, 18). Using chemically synthesized nepetalactol, we demonstrated that the silver vine response is regulated via μ-opioid receptors that are involved in rewarding and euphoric effects in humans. The rubbing and rolling response transfers nepetalactol from the plant leaves onto the cat’s face and head where it acts as a mosquito repellent, finally revealing the likely biological significance of this enigmatic feline behavior, first observed more than 300 years ago.
RESULTS
Nepetalactol is a potent stimulant for silver vine response
Previous attempts to isolate bioactive compounds from dried leaves of silver vine used steam distillation, alkaline heat treatment, and acid treatment (13–15), all of which have the potential to decompose bioactive components. To avoid this problem, an organic solvent extract from silver vine leaves was resolved into six fractions using silica gel normal-phase column chromatography (Fig. 1A, step 1). The bioactivity of each of these fractions, corresponding to 1.2 g of leaves, was tested using four cats that responded positively to the unfractionated leaf extract (Fig. 1B). Only fraction 3 eluted by n-hexane/ethyl acetate (80:20, v:v) and fraction 4 eluted by n-hexane/ethyl acetate (70:30) induced face rubbing and rolling over in four and three subject cats, respectively. Although fraction 3 stimulated a more prolonged response than fraction 4 in all subjects (Fig. 1C), gas chromatography/mass spectrometry (GC/MS) analysis revealed that compounds with known bioactivity (isoiridomyrmecin, dihydronepetalactone, and isodihydronepetalactone) were at markedly lower levels in fraction 3 compared to fraction 4 (Fig. 1D). This suggests an important contribution of one or more unidentified compounds in fraction 3, which induce the behavioral response. To identify these unknown compounds, bioactive components in fraction 3 were further purified by normal-phase and reversed-phase high-performance liquid chromatography (HPLC) and finally enriched into a bimodal peak by HPLC (Fig. 1A, steps 2 to 5, and movie S1).

GC/MS of the final bioactive fraction (fraction 3-3-2-3) detected five major peaks (Fig. 1E); one, at 43.7 min, had a unique mass spectrum that matched (87%) a standard spectrum of nepetalactol (fig. S1A) in the Wiley MS library. Nepetalactol (Fig. 1F) is a common important biosynthetic precursor of iridoid monoterpenes (19) and shares a very similar structure with cis-trans nepetalactone (Fig. 1F) except for lactol and lactone moieties. A recent study also identified nepetalactol from silver vine leaves but did not examine its bioactivity in cats (20). We therefore synthesized cis-trans nepetalactol for further investigation. The mass spectrum of the 43.7-min peak had 98% similarity with authentic nepetalactol. Further, fraction 3-3-2-3 spiked with authentic nepetalactol yielded a single coeluting GC/MS peak with the same retention index (RI = 2078) as authentic nepetalactol alone (RI = 2080; fig. S1B). These results provide clear evidence that the peak at 43.7 min was cis-trans nepetalactol. We conjectured that the other two peaks (at 39.5 and 39.7 min) were stereoisomers of iridodial (Fig. 1F); the mass spectra (fig. S1C) were in good agreement with published spectra (21, 22). As iridodial has a readily epimerizing dialdehyde structure and is easily oxidized under atmospheric conditions (23), we thought that iridodial is an unsuitable stimulant for a reliable behavioral assay. Thus, further behavioral assays evaluated the bioactivity of chemically synthesized nepetalactol.
Bioactivity of nepetalactol in felid and nonfelid species
In this study, we used 25 laboratory cats that consisted of 18 positive and 7 negative responders to silver vine leaf extract (table S1). In behavioral assays using 15 of the 18 positive responder cats, all subjects exhibited face rubbing and rolling over in response to 50 μg of nepetalactol-impregnated filter paper (nepetalactol-paper); most of them then lost interest in the paper within 10 min after presentation (Fig. 2, A and B, and movie S2), very similar to the behavioral response toward unextracted plant materials (6). No cats exhibited a flehmen-like response, which is a functional behavior that transfers compounds such as pheromones from the oral cavity to the sensory vomeronasal organs (24). The duration of the behavioral response to nepetalactol-paper was more prolonged than that to control solvent filter paper (control-paper) presented simultaneously on the floor (Wilcoxon matched-pair test, one-tailed P = 0.0003; Fig. 2C). To test the generality of the bioactivity of nepetalactol, we also tested this similarly in 30 free-ranging feral cats using nepetalactol-paper versus control-paper. Seventeen of the 30 cats (57%) rubbed their faces against at least one paper. Almost all face rubbing and rolling among these 17 feral cats was directed toward the nepetalactol-paper such that the overall response toward nepetalactol-paper was substantially more prolonged than toward control-paper (P = 0.0001; Fig. 2, D and E, and movie S2), similar to laboratory cats. The other 13 cats did not respond to either papers, suggesting that they either were inherent negative responders (5) or just were not responsive in an unfamiliar test situation.

Next, we compared the behavioral responses of 12 of the 18 positive responder cats to each of the known bioactive iridoids. Nepetalactol (silver vine) and nepetalactone (catnip) induced more prolonged face rubbing and rolling than other iridoids (Fig. 2F; χ2 = 24.0, df = 5, P = 0.0002). At least one cat did not exhibit the characteristic response toward each iridoid except for nepetalactol. Further, the nepetalactol content of silver vine leaves (20.71 μg/g of wet weight) was much higher than isoiridomyrmecin (1.42 μg/g), iridomyrmecin (below the GC/MS detection limit), dihydronepetalactone (<0.18 μg/g), or isodihydronepetalactone content (<0.18 μg/g). Considering also that fraction 3 had stronger bioactivity than fraction 4, as shown in Fig. 1C, and nepetalactol had bioactivity in all of the 18 positive responder cats tested (Fig. 2, C and F), nepetalactol is the most potent and major bioactive iridoid in silver vine leaves. We concluded that nepetalactol is the most suitable stimulant for a reliable and reproducible behavioral assay.
Nondomesticated captive felids tested at zoos in Japan (an Amur leopard, P. pardus orientalis; two jaguars, P. onca; two Eurasian lynx, L. lynx) also exhibited more prolonged face rubbing and rolling on nepetalactol-paper than on control-paper (exact P = 0.031; Fig. 2, G and H, and movie S3), a bias that did not differ significantly from the laboratory cats (Mann-Whitney U test of bias; z = 1.27, P = 0.23).
We also tested the response of domestic dogs (Canis lupus familiaris, n = 8) and laboratory mice (C57BL/6 or BALB/cAJcl strain males, n = 10) to nepetalactol. All animals tested were uninterested in nepetalactol, and none exhibited a silver vine response (movie S3). The lack of response to nepetalactol in dogs and mice differed substantially from the positive response found among 72% of the 25 laboratory cats tested in this study (Fisher’s exact tests, domestic dogs: P < 0.0005; mice: P < 0.0005).
Activation of the μ-opioid system during silver vine response in cats
Subjective observations of the behavioral response of cats to catnip suggest that they may experience a positive reaction that has often been interpreted as extreme pleasure (17, 25). Thus, we hypothesized that olfactory reception of nepetalactol stimulates the μ-opioid system, which controls rewarding and euphoric effects in humans (26). First, we examined temporal changes in plasma levels of β-endorphin (a peptide hormone and an endogenous opiate) in five cats 5 min before and after exposure to 200 μg of nepetalactol (corresponding to the contents in approximately 10 leaves) on day 1 (inducing the behavioral response) and then to a blank stimulus control 4 days later (Fig. 3A). Plasma β-endorphin concentration was markedly elevated after exposure to nepetalactol but not after exposure to a control stimulus
[repeated-measures analysis of variance (ANOVA), interaction between
stimulus and time point: F1,4 = 9.97, P = 0.034; Fig. 3B]
.

To test whether the μ-opioid system is directly involved in regulation of the behavioral response to nepetalactol, we examined the behavioral response to nepetalactol in six cats that had been administered saline (day 1) or naloxone (day 2), an antagonist of μ-opioid receptors (Fig. 3C). While all cats exhibited a typical response to nepetalactol-paper after saline administration, the duration of their characteristic rubbing and rolling response was reduced significantly after naloxone administration on the following day (Wilcoxon matched-pair test, two-tailed exact P = 0.031; Fig. 3D and movie S4). By contrast, six cats administered saline on both days as a control showed no reduction in response to nepetalactol (P = 1.00; Fig. 3E), differing significantly from the reduced response caused by naloxone (Mann-Whitney test of change between day 1 and day 2; P = 0.04). Naloxone did not affect the duration of other activities compared to vehicle alone (walking: P = 0.31; grooming: P = 0.31; fig. S2, A and B), confirming that the naloxone dose administered did not disturb locomotor activities or motor functions during the observations. Inhibition of the μ-opioid system specifically suppressed the rubbing and rolling response in the cats. These results demonstrate that the μ-opioid system is involved in the induction of the feline behavioral response.
Mosquito-repellent activity of nepetalactol
The consistent expression of such a characteristic response to nepetalactol suggests that the response has an important adaptive function for cats. On the basis of reports that nepetalactone from catnip has mosquito-repellent activity when applied to humans (27–29), we hypothesized that the characteristic rubbing and rolling against plants allows cats to transfer nepetalactol or nepetalactone onto the fur for chemical defense against mosquitoes and possibly also against other biting arthropods. In this study, we tested whether nepetalactol is repellent to Aedes albopictus, a mosquito common in Japan and China (30). A. albopictus avoided both silver vine leaves (five leaves, containing approximately 100 μg of nepetalactol) and nepetalactol alone (50 μg, 200 μg, and 2 mg) compared to a solvent control, when each was placed separately into test cages that had shelters into which A. albopictus could move (Fig. 4A; ANOVA, effect of stimulus F4,15 = 79.93, P < 0.0001; planned contrasts confirmed that avoidance of each test stimulus was significantly greater than the control, P < 0.003; Fig. 4B). This indicates that nepetalactol acts as a repellent against A. albopictus, consistent with the previously reported repellent activity of nepetalactone (29).

The function of rubbing behavior in nepetalactol-stimulated cats
Next, we examined whether the characteristic rubbing and rolling response functions to transfer nepetalactol to the cat’s face, head, and body. To establish the importance of contact with the source (to rub nepetalactol onto the fur), seven laboratory cats were tested with 200 μg of nepetalactol versus control on papers placed on the test cage walls or ceiling. In this arrangement, cats could rub the papers with their faces, but rolling would not allow rubbing contact with the stimulus. As expected, all subjects rubbed their faces and heads on nepetalactol-paper placed on the cage walls more frequently than on control-paper (Wilcoxon matched-pair test, exact P = 0.008; Fig. 5A and movie S5). When papers were more difficult to contact on the cage ceiling, five of seven subject cats stood on their hind legs, held on to the ceiling mesh with their fore paws, and rubbed their faces and heads on nepetalactol-paper more frequently than on control-paper (exact P = 0.031; Fig. 5B and movie S5). However, no subject cat rolled on the ground when test papers were on the cage walls or ceiling, in stark contrast to typical rolling observed in all subjects when filter papers were placed on the floor (χ2 = 29.0, df = 1, P < 0.0001). Strong motivation to contact nepetalactol was further evidenced in high-ceiling cages, when two of seven subjects climbed the 116-cm walls to reach nepetalactol-paper on the ceiling and then proceeded to rub their faces and heads on nepetalactol-paper as before (Fig. 5C and movie S6). Thus, rubbing is specifically targeted at the nepetalactol source.

Rubbing against the source (200 μg of nepetalactol) should transfer the material to the fur, but the quantity of nepetalactol in ethanol-soaked cotton used for wiping the face and head fur was below the limit of detection (2.2 μg) by our experimental procedure using GC/MS as a detector, indicating that no more than 1% of the material was recovered on the cotton wipe. To provide a more sensitive test, subject cats were tested with face and head wipes from donors that had rubbed nepetalactol-papers with or without direct physical contact versus wipes from unstimulated donors (Fig. 5D). We predicted that subjects would detect nepetalactol on papers used to wipe donors that had contact-rubbed nepetalactol-paper, but they would not respond to wipes from unstimulated donors or from donors that rubbed in response to nepetalactol when they could not physically contact the source. In agreement with our prediction, subjects rubbed and rolled only in response to wipes from donors that had physically contact-rubbed nepetalactol-papers (Fig. 5E; interaction between donor nepetalactol stimulation and direct contact, F1,4 = 9.97, P = 0.034; behavioral response to donor with nepetalactol physical contact, F1,4 = 12.35, P = 0.025; behavioral response to donor without nepetalactol physical contact, F1,4 = 0.32, P = 0.60). Thus, face rubbing transfers nepetalactol onto the cat’s fur.
Silver vine response provides cats with mosquito repellency
To examine whether nepetalactol on the fur protects cats from mosquito bites, the heads of six pairs of anesthetized cats were placed into opposite sides of a test cage. One cat’s head had been treated with nepetalactol (500 μg) and the other with the appropriate solvent control. The number of A. albopictus landing on the nepetalactol-treated head was half the number landing on the control head on average (repeated-measures ANOVA, F1,5 = 8.56, P = 0.033; Fig. 5F), showing significant repellence. Lastly, to investigate a more natural situation, we assessed whether cats responding to silver vine leaves transfer sufficient active compound(s) to repel A. albopictus, compared to unstimulated cat controls in the same two-head test. Cats that had rubbed against silver vine leaves were significantly avoided by A. albopictus compared to control cats that had not been stimulated by the leaves (repeated-measures ANOVA, F1,5 = 11.78, P = 0.019; Fig. 5G). By contrast, there was no significant difference in the number of A. albopictus landing on the head of control cats versus cats that had not rubbed when presented with silver vine leaves (F1,5 = 0.029, P = 0.87; Fig. 5H; difference in bias between tests, F1,10 = 9.21, P = 0.013). These results show that nepetalactol, transferred to face and head fur by rubbing against silver vine leaves, functions as a repellent against A. albopictus in cats. This is convincing evidence that the characteristic rubbing and rolling response functions to transfer plant chemicals that provide mosquito repellency to cats.
DISCUSSION
This study has found that the iridoid nepetalactol is the major bioactive compound in the leaves of silver vine that induces characteristic rubbing and rolling in cats (Fig. 6). Further, nepetalactol had similar bioactivity in Amur leopard, jaguar, and Eurasian lynx. As most of the Felidae species so far tested have shown positive responses toward catnip (13 of 21 species tested from a total of 41 living species in this family) (2–4), it is likely that this characteristic response to nepetalactol will also be common across many of the Felidae. Using synthesized nepetalactol, we have demonstrated that the μ-opioid system regulating euphoric and rewarding effects in humans (26) is involved in the expression of the catnip and silver vine response in cats. We have uncovered an adaptive benefit of the behavioral response in cats: Rubbing and rolling on the leaves of silver vine transfers nepetalactol to the heads, faces, and bodies of cats. As a consequence, this reduces the number of A. albopictus mosquitoes that land on the animal’s head, helping to protect from mosquito bites. These findings provide new insight into this well-known and characteristic plant-induced feline response, for which the biological function was first questioned in popular science culture more than 300 years ago.

We have yet to understand fully how the μ-opioid system is activated by nepetalactol in cats. In previous reports, essential oils from N. caesarea, of which nepetalactone is a major component, had an analgesic effect involving μ-opioid receptors in rats, but these compounds were injected intraperitoneally (31). Oral administration of nepetalactone to cats has no marked physiological effects (16). Further, cats do not require naso-oral contact with nepetalactol-paper for this to stimulate the silver vine response. This suggests that nepetalactol and other bioactive iridoids activate the μ-opioid system via chemical sensing through the olfactory system for the response. The vomeronasal system does not appear to be involved in inducing the response in cats (6). Previous studies have reported functional connections between the olfactory and opioidergic processes in mammals such as rats (32–35), suggesting that cats may have neural circuitry that connects the olfactory neurons that detect bioactive iridoids with the μ-opioid system. While our results indicate that taste is not essential for the silver vine response, cats exhibiting the response commonly lick the stimulus when this is possible. As previous studies have reported that tastes and other orosensory stimulation can activate β-endorphin release in mammals (36, 37), the possibility cannot be excluded that the taste system also participates in regulating the silver vine response in cats.
The feline response to specific chemicals in plant materials is nonaddictive (38). This may be because the μ-opioid system is stimulated by an increase in endogenous β-endorphin secretion when olfactory neurons are activated by these iridoids. This contrasts with the development of addiction to exogenous opiates such as morphine in mammals including cats (39), when μ-opioid receptors are directly activated by opiates via the bloodstream (40, 41).
Although we only tested for a repellent effect on A. albopictus in this study, we might also expect nepetalactol to be repellent to other mosquito species including A. aegypti, which is a common vector of yellow fever, dengue, and Zika viruses (42), consistent with the broad repellence of nepetalactone across a range of mosquito and other biting arthropods (27–29). Our findings suggest that nepetalactol may be a new natural candidate repellent to help reduce mosquito problems in human society.
We propose that silver vine and catnip response provides repellency against A. albopictus by transferring nepetalactol or nepetalactone from plants onto a cat’s fur. Face rubbing against plant sources of the repellent will help to protect the face and head of the animal, as the mouth, eyelids, ears, and nose of felines have relatively little fur and are therefore easy targets for mosquitoes. Although the rolling response following face rubbing, which exposes the belly, may look like a defenseless behavior, it enables cats to pick up repellent iridoids on other areas of their bodies. Notably, cats did not roll on the ground when stimuli were placed such that rolling would not bring the cat into contact with the stimulus. Therefore, rolling is a functional behavior rather than an indicator of euphoria or extreme pleasure.
The silver vine and catnip response is an important example of how animals use plant metabolites for protection against insect pests. There are other examples that nonhuman animals may exploit some chemicals emitted from other species for protection against insect pests: boat-tailed grackles (Quiscalus major) and white-nosed coatis (Nasua narica) rub fruits of Citrus spp. against themselves (43), chimpanzees (Pan troglodytes schweinfurthii) use sleeping platforms created from specific trees as a source of repellents (44), house sparrows (Passer domesticus) and house finches (Carpodacus mexicanus) living in urban habitats bring cigarette butts to the nest (45), and capuchin monkeys (Cebus olivaceus) anoint themselves with millipedes (Orthoporus dorsovittatus) (46). Each species may select plants and other materials as insect repellents during evolution. The examples so far uncovered of animals self-anointing or using prophylactic self-medication (47) with secondary plant metabolites to protect against pests and diseases typically occur in individual species, although the same pests and diseases may affect many species. However, self-anointing with plant iridoids is common in Felidae, although not in the Carnivora more generally, suggesting that the behavior first evolved in a common felid ancestor and has been retained. As many felids rely on stealth to stalk and ambush their prey, requiring them to remain cryptic and often unmoving, a repellent that reduces their susceptibility to both the irritation of biting mosquitoes and the diseases that these insect vectors carry is likely to provide a strong selective advantage. Stimulation of the μ-opioid system might further help by providing analgesia to reduce irritation where biting arthropods have not been repelled. While this can explain why this characteristic behavior has been retained in many Felidae species, it does not explain why the behavior has evolved only in felids. A specific ability to detect these iridoid chemicals in a common felid ancestor may have been a crucial preadaptation that provided the opportunity for this self-anointing behavior to evolve, allowing animals from multiple species within this family to acquire mosquito repellence.
The catnip response is inherited as an autosomal dominant trait in domestic cats (5), strongly suggesting the presence of one or few genes responsible for the silver vine and catnip response in felids. The felids that were positive responders in this study focused close attention toward nepetalactol and other iridoid stimuli presented, responding even to the low level of nepetalactol recovered by wiping donors that had rubbed this into their fur during the silver vine response. By contrast, dogs, mice, and negative responder cats failed to even stop and sniff nepetalactol stimuli. These findings suggest that domestic cats and the nondomestic felids that also respond might have acquired specific olfactory receptor(s) that detect nepetalactol and other iridoids emitted from some plants with high sensitivity. A genome-wide association study among positive and negative responder cats to identify the olfactory receptor genes and specific neuronal pathways involved in this response could provide invaluable clues for understanding how and why this characteristic response to silver vine and catnip has evolved specifically in felids.
MATERIALS AND METHODS
Animals
Twenty-five healthy mixed-breed laboratory cats (aged 1 to 16 years; 7 intact males, 15 intact females, and 3 spayed females) were used in this study. The cats were maintained at 24°C under a 12-hour/12-hour light/dark photoperiod (lights on at 7:00 a.m.). The male cats were housed individually in three-storied cages (93 cm × 63 cm × 178 cm). Female cats were housed in pairs or individually in three-storied cages. The cats had continuous access to drinking water, received dry food produced by Royal Canin (Aimargues, France) twice daily (morning and evening), and had continuous visual, auditory, and olfactory contact with others in the room. Table S1 shows the experiments that each cat participated in. Thirty feral cats of unknown sex and age were tested in the field at Tashirojima in Ishinomaki, Miyagi Prefecture, Japan. Additional nondomestic felids were tested at Osaka Municipal Tennoji Zoological Gardens (Osaka, Japan) and Kobe Oji Zoo (Kobe, Japan): two jaguars (P. onca, one male and one female), two Eurasian lynxes (L. lynx, one male and one female), and an Amur leopard (P. pardus orientalis, male).
Eight domestic dogs (C. lupus familiaris; aged 1 to 12 years; two male and three female border collies, one female Shih Tzu, one female Maltese, and one female mixed breed) were tested in this study. They were kept as companion animals by a variety of owners and thus varied in diet and individual experience. Ten laboratory mice (four male C57BL/6 and six male BALB/cAJcl, aged 8 weeks) were purchased from Japan CLEA (Shizuoka, Japan) for behavioral assays. Mice of the same strain were housed in the same cage (235 cm × 353 cm × 160 cm) under a regular 12-hour/12-hour light/dark photoperiod with food and water ad libitum. All procedures followed local and national animal ethics guidelines and were approved by the animal research committee of Iwate University (approval numbers A201614, A202027, A202038, and A202057).
Chemicals
Chloroform (HPLC grade, ≥99.7% purity), methanol (HPLC grade, ≥99.7% purity), n-hexane (HPLC grade, ≥96.0% purity), ethyl acetate (99.5% purity), diethyl ether (reagent grade, ≥99.5% purity), and ethanol (reagent grade, 99.5% purity) were purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan). Naloxone hydrochloride dihydrate (>98% purity) and saline The effect of this erectile dysfunction drug lasts cialis samples free for more than 4 hours. If you are buying cheapest viagra in australia then you should choose the one which ensure guaranteed satisfaction. Also known by the name of Impotence, Erectile Dysfunction (ED) is an incapability to attain viagra 100mg pfizer http://www.molineanimalaid.org/New%20News.pdf or maintain an erection. He concludes: “My paintings are a visual expression of my thoughts, feelings, moods and memories — or what I had of course failed to tell her, is soft viagra tabs that for the last couple of years. were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Otsuka Pharmaceutical Factory Inc. (Tokushima, Japan), respectively.
Cis-trans nepetalactol, iridomyrmecin, and isoiridomyrmecin were synthesized according to previous reports (48, 49). Cis-trans nepetalactone, dihydronepetalactone, and isodihydronepetalactone were synthesized as described below.
Cis-trans nepetalactone. To a solution of nepetalactol (22.1 mg, 0.131 mmol) in dry benzene (5.2 ml) was added silver carbonate on Celite [50 weight % (wt %), 145 mg, 0.263 mmol] under argon. The reaction mixture was allowed to warm to 90°C and stirred for 30 min. The reaction mixture was filtered through a pad of Celite. The filtrate was concentrated to dryness in vacuo. The residue was purified by column chromatography (neutral silica gel, n-hexane/diethyl ether 9:1) to give nepetalactone (19.8 mg, 11.9 mmol, 91%) as a colorless oil.
[α]24D +20.3 (c 0.975, Et2O); infrared (IR) (film) νmax 2958, 2930, 2871, 1749, 1687, 1453, 1384, and 825 cm−1; 1H nuclear magnetic resonance (NMR) (400 MHz, CDCl3) δ 1.19 (3H, d, J = 6.5 Hz, MeCHCHC═O), 1.21 to 1.27 (1H, m, CHAHBCH2CHC═C), 1.50 to 1.59 (1H, m, CHAHBCH2CHC═C), 1.62 (3H, s, MeC═C), 1.83 to 1.94 (1H, m, CHC═O), 1.97 to 2.08 (1H, m, CHAHBCHC═C), 2.37 (1H, quint, J = 7.5 Hz, MeCHCHC═O), 2.33 (1H, t, J = 9.0 Hz, CHAHBCHCMe═C), 2.74 (1H, q, J = 8.0 Hz, CHC═C), and 6.17 (1H, t, J = 1.0 Hz, MeC═CH); 13C NMR (100 MHz, CDCl3) δ 15.5, 20.3, 29.7, 30.9, 33.0, 39.7, 40.7, 49.3, 115.3, 133.6, and 170.9; electrospray ionization (ESI)/high-resolution MS (HRMS) for C10H14O2Na [M+Na]+ calculated 189.0891, found 189.0892.
Dihydronepetalactone. To a solution of nepetalactone (55.4 mg, 0.334 mmol) in ethanol (7 ml) was added palladium on carbon (10 wt %, 22.1 mg) under argon at room temperature. The reaction mixture was stirred for 3 hours under hydrogen gas and filtered through a pad of Celite. The filtrate was concentrated to dryness in vacuo. The residue was purified by column chromatography (two times, neutral silica gel, n-hexane/diethyl ether 7:1 and neutral flash silica gel, n-hexane/diethyl ether 9:1) to give dihydronepetalactone (38.9 mg, 0.231 mmol, 69%) as a colorless oil.
[α]D29 +54.9 (c 0.995, CHCl3); IR (film) νmax 2963, 1731, 1456, and 1377 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.90 (3H, d, J = 7.0 Hz, CHMeCH2O), 1.20 (3H, d, J = 6.5 Hz, CHMeCHC═O), 1.20 (1H, m, CHAHBCH2CHCHMeCH2O), 1.43 (1H, tdd, J = 12.5, 10.5, 6.0 Hz, CHAHBCHCHMeCH2O), 1.74 (1H, quint, J = 7.0 Hz, CHAHBCHCHMeCH2O), 1.93 (1H, sext, J = 6.0 Hz, CHAHBCH2CHCHMeCH2O), 2.00 (1H, m, CHMeCH2O), 2.24 (1H, tq, J = 11.0, 7.0 Hz, CHMeCH2O), 2.42 (1H, dd, J = 11.0, 9.5 Hz, CHC═O), 2.52 (1H, m, CHCHMeCH2O), 4.02 (1H, ddd, J = 11.0, 4.0, 1.5 Hz, CHAHBO), and 4.08 (1H, t, J = 11.0 Hz, CHAHBO); 13C NMR (100 MHz, CDCl3) δ 13.0, 19.3, 26.3, 30.9, 35.0, 40.4, 41.5, 69.9, and 174.3; ESI/HRMS for C10H16O2Na [M+Na]+ calculated 191.1048, found 191.1044.
Isodihydronepetalactone. To a solution of aminal (139 mg, 0.541 mmol) in ethanol (10 ml) was added palladium on carbon (10 wt %, 55.7 mg) under argon at room temperature. The reaction mixture was stirred for 24.5 hours under hydrogen gas and filtered through a pad of Celite. The filtrate was concentrated to dryness in vacuo. The residue was purified by column chromatography (neutral silica gel, n-hexane/diethyl ether 49:1 → 19:1) to give reduced aminal (93.5 mg, 0.360 mmol, 67%) as a colorless oil.
[α]D22 −10.7 (c 0.950, CHCl3); IR (film) νmax 2949, 2870, 1599, 1503, 1456, 835, 752, and 693 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.98 (3H, d, J = 6.5 Hz, CHMeCHCHN), 1.10 (3H, d, J = 7.0 Hz, CHMeCH2O), 1.18 (1H, m, CHAHBCHCHMeCH2O), 1.55 to 1.72 (1H, m, CHAHBCHCHMeCH2O, CHMeCH2O), 1.77 to 1.98 (3H, m, CHAHBCH2CHCHMeCH2O, CHCHMeCH2O, CHMeCHCHN), 2.00 to 2.15 (2H, m, CHCHN, CHAHBCH2CHCHMeCH2O), 2.99 (3H, s, NMe), 3.53 (1H, dd, J = 11.0, 4.5 Hz, CHAHBO), 3.81 (1H, dd, J = 11.0, 3.5 Hz, CHAHBO), 4.68 (1H, d, J = 8.5 Hz, CHCHN), 6.82 (1H, t, J = 7.0 Hz, aromatic), 6.93 (2H, d, J = 8.0 Hz, aromatic), and 7.25 (2H, dd, J = 9.0, 7.0 Hz, aromatic); 13C NMR (100 MHz, CDCl3) δ 18.8, 21.4, 29.5, 30.8, 32.5, 33.0, 35.4, 43.7, 45.5, 68.1, 88.2, 115.4, 118.5, 128.9, and 150.7; ESI/HRMS for C17H25NONa [M+Na]+ calculated 282.1828, found 282.1827.
To a solution of reduced aminal (93.5 mg, 0.363 mmol) in tetrahydrofuran (3.2 ml) and water (0.4 ml) was added p-toluenesulfonic acid monohydrate (69.1 mg, 0.363 mmol) at room temperature. The reaction mixture was stirred for 1 hour and diluted with diethyl ether (10 ml). The resulting solution was washed with saturated aqueous sodium bicarbonate (5 ml × 1) and brine (5 ml × 1) and then dried over sodium sulfate. The solution was concentrated to dryness in vacuo. The residue was purified by column chromatography (neutral silica gel, n-hexane/diethyl ether 9:1 → 7:1) to give dihydronepetalactol (53.9 mg, 0.316 mmol, 87%) as an inseparable diastereomeric mixture (dr = 10:1 by 1H NMR) as a colorless oil.
[α]D27 +22.2 (c 0.605, CHCl3); IR (film) νmax 3388, 2947, 1458, 1387, 1375, 1362, 1275, 1158, 1115, 1073, and 1044 cm−1; (major) 1H NMR (400 MHz, CDCl3) δ 0.79 (3H, d, J = 6.5 Hz, CHMeCHO), 1.01 (3H, d, J = 6.5 Hz, CHMeCHCHOH), 1.20 (1H, m, CHAHBCH2CHCHMeCH2O), 1.40 to 1.55 (3H, m, CHCHOH, CHMeCH2O, CHAHBCHCHMeCH2O), 1.70 (1H, dquint, CHAHBCHCHMeCH2O), 1.82 to 2.07 (3H, m, CHCHMeCH2O, CHAHBCH2CHCHMeCH2O, CHMeCHCHOH), 2.72 (1H, br s, OH), 3.41 (1H, dd, J = 11.5, 5.0 Hz, CHAHBO), 3.58 (1H, t, J = 11.0 Hz, CHAHBO), and 5.13 (1H, s, CHOH); 13C NMR (100 MHz, CDCl3) δ 15.4, 19.8, 27.8, 30.8, 31.5, 33.6, 41.4, 50.9, 64.2, and 92.9; (minor): 1H NMR (400 MHz, CDCl3) δ 0.76 (d, J = 6.5 Hz, CHMeCHO), 1.11 (3H, d, J = 6.5 Hz, CHMeCHCHOH), 2.27 (1H, m, CHMeCHCHOH), 3.06 (1H, t, J = 11.5 Hz, CHAHBO), 2.17 (1H, br s, OH), 3.82 (1H, dd, J = 11.5, 5.0 Hz), and 5.02 (1H, dd, J = 5.0, 2.5 Hz, CHOH); 13C NMR (100 MHz, CDCl3) δ 14.9, 22.8, 28.4, 30.0, 30.2, 32.5, 46.7, 51.3, 71.2, and 97.1.
To a solution of dihydronepetalactol (52.7 mg, 0.310 mmol) in dry benzene (12 ml) was added silver carbonate on Celite (50 wt %, 341 mg, 0.620 mmol) under argon. The reaction mixture was allowed to warm to 90°C and stirred for 30 min. The reaction mixture was filtered through a pad of Celite. The filtrate was concentrated to dryness in vacuo. The residue was purified by column chromatography (neutral silica gel, n-hexane/ethyl acetate 4:1) to give isodihydronepetalactone (49.3 mg, 0.293 mmol, 95%) as a colorless oil. [α]D29 −0.4 (c 1.00, CHCl3); IR (film) νmax 2955, 2871, 1730, 1458, and 1394 cm−1; 1H NMR (400 MHz, CDCl3) δ 0.99 (3H, d, J = 6.5 Hz, CHMeCH2O), 1.20 (3H, d, J = 6.0 Hz, CHMeCHC═O), 1.10 to 1.31 (2H, m, CHAHBCH2CHCHMeCH2O, CHAHBCHCHMeCH2O), 1.61 (1H, m, CHMeCH2O), 1.86 (1H, quint, J = 6.0, 2.0 Hz, CHAHBCH2CHCHMeCH2O), 1.98 to 2.15 (2H, m, CHCHMeCH2O, CHAHBCHCHMeCH2O), 2.25 (1H, m, CHMeCHC═O), 2.34 (1H, dd, J = 10.5, 8.5 Hz, CHC═O), 3.87 (1H, t, J = 10.5 Hz, CHAHBO), and 4.15 (1H, dd, J = 10.5, 3.5 Hz, CHAHBO); 13C NMR (100 MHz, CDCl3) δ 15.7, 20.2, 31.8, 34.3, 35.1, 38.7, 44.6, 49.0, 72.8, and 175.0; ESI/HRMS for C10H16O2Na [M+Na]+ calculated 191.1048, found 191.1045.
Identification of nepetalactol from silver vine leaves
Fresh leaves were sampled from wild populations of silver vine grown outdoors during June and July in 2016, 2019, and 2020 at Takizawa, Kunohe, and Morioka (Iwate prefecture, Japan). Total lipids were extracted from the leaves using the Folch method within 3 hours from sampling. Briefly, the leaves were soaked in a 20-fold (w/v) organic solvent of chloroform and methanol (2:1, v/v) at 15°C. After removal of the leaf residue by filtration through paper, the solvent of the leaf extract was removed by rotary evaporation, and the extract was dissolved in n-hexane to a concentration equivalent to 6 g of wet leaves per milliliter.
To select positive responders among 25 laboratory cats, filter papers (Advantec qualitative no. 1, 70 mm; Toyo Roshi Kaisha Ltd., Tokyo, Japan) were attached to the bottom of petri dishes (9 cm diameter; AS ONE Corp., Osaka, Japan) using double-sided adhesive tape. The filter papers were impregnated with 33 μl of leaf extract (corresponding to 0.2 g of silver vine leaves) or the same volume of n-hexane as a negative control. Laboratory cats were put individually in test cages (88 cm × 57 cm × 70 cm or 93 cm × 63 cm × 116 cm) for habituation 5 min before assays. Immediately before the assays, two petri dishes were fixed on the left and right side of the cage floor (randomly assigned) with double-sided adhesive tape and then the lids were removed. The cats’ behavioral responses were recorded using a digital video camera (Handycam HDR-CX560V; Sony, Tokyo, Japan) placed in front of the cage until at least approximately 5 min after the cats showed disinterest in the dishes. Bioactivity was evaluated as being positive when a subject exhibited face rubbing and/or rolling-over behavior directed to a dish. On the basis of responsiveness, the 25 cats were separated into 18 positive (72%) and 7 negative responders (28%).
Four of the 18 positive responders were used to test bioactivity during purification of bioactive compounds from the extract. In purification step 1, normal-phase column chromatography was performed with a 40-ml silica gel column (Iatrobeads 6RS-8060; LSI Medience Co., Tokyo, Japan) to fractionate 20 ml of extract (corresponding to 120 g of silver vine leaves) using stepwise elution; 100 ml of solvent was used for each fraction, and the mixing ratios (v/v) of n-hexane/ethyl acetate were 100:0, 90:10, 80:20, 70:30, and 60:40 for fractions 1 to 5, respectively, followed by 100 ml of methanol for fraction 6. To identify the fraction with bioactivity for cats, each fraction (200 μl of aliquots; corresponding to 1.2 g of leaves) was pipetted onto a filter paper fixed to the bottom of separate petri dishes, and the n-hexane solvent was allowed to evaporate under atmospheric conditions. Immediately before the assays, the six covered petri dishes containing the six fractions were fixed with double-sided adhesive tape to the floor of each cage, ordered from fraction 1 (left) to 6 (right), and then the lids were removed. Bioactivity of each fraction was evaluated as the duration of face rubbing and rolling over, using Behavioral Observation Research Interactive Software (BORIS) version 7.9.8 (50).
In purification step 2, 80 ml of fraction 3 prepared in step 1 was concentrated by rotary evaporation to 5 ml and then injected (4 ml of aliquot) into a normal-phase HPLC apparatus equipped with a silica gel column (Senshu Pak Pegasil Silica, 10 mm internal diameter × 250 mm; Senshu Scientific Co., Tokyo, Japan). Separation was performed by isocratic elution with n-hexane/ethyl acetate = 80/20 (3 ml/min of flow rate). The HPLC effluent was partitioned into five fractions (3-1 to 3-5) depending on color differences (fraction 3-1 was 28 ml, the other fractions were 4 ml). Fraction 3-1 was concentrated by rotary evaporation to 4 ml. The bioactivity of each fraction (40 μl of aliquot) was tested as described above.
In purification step 3, 4 ml of fraction 3-2 prepared in step 2 was concentrated by nitrogen evaporation to 400 μl and then injected (160 μl of aliquot) into a reversed-phase HPLC equipped with a C22 column (Senshu Pak Docosil, 10 mm internal diameter × 250 mm; Senshu Scientific Co.). Separation was performed by isocratic elution with methanol (5 ml/min of flow rate). The HPLC effluent was introduced into an inline ultraviolet detector (220 nm) and divided into six fractions (3-3-1 to 3-3-6). HPLC was repeated twice under the same condition, and then each fraction was combined into a glass tube. Each fraction was concentrated by nitrogen evaporation to 2 ml, and the bioactivity was examined (using 10 μl of aliquots) as described above.
In purification step 4, 2 ml of fraction 3-3-2 prepared in step 3 was concentrated by nitrogen evaporation to 200 μl and then injected (100 μl of aliquot) into a reversed-phase HPLC equipped with a C30 column (Develosil C30-UG-5, 4.6 mm internal diameter × 250 mm; Nomura Chemical Co. Ltd., Aichi, Japan). Separation was performed by isocratic elution using methanol (1 ml/min of flow rate). The HPLC effluent was passed through an inline ultraviolet detector (220 nm) and partitioned into seven fractions (3-3-2-1 to 3-3-2-7). HPLC was repeated twice under the same condition, and then each fraction was combined into a glass tube. Each fraction was concentrated by nitrogen evaporation to 400 μl, and the bioactivity of each fraction (10 μl of aliquot) was examined as described above.
Final step 5 confirmed the purity of fraction 3-3-2-3 prepared in step 4. Reversed-phase HPLC with a C30 column was used to analyze 100 μl of fraction 3-3-2-3 using the same HPLC conditions as in step 4.
High-performance thin-layer chromatography analysis
Thin-layer chromatographic analysis was performed on a silica gel high-performance thin-layer chromatography (HPTLC) plate (HPTLC Silica gel 60; Merck Millipore Co., Darmstadt, Germany). The unfractionated leaf extract and each fraction in purification step 1 and step 2 were developed with a solvent system of n-hexane/ethyl acetate (80/20, v/v). After the solvent was air-dried, spots were visualized by spraying with 3% (w/v) copper acetate in 8% (w/v) phosphoric acid solution and heating at 180°C.
Gas chromatography/mass spectrometry
All GC/MS analyses were carried out using a QP2010 Ultra device (Shimadzu Co., Kyoto, Japan) operated in electron impact mode (70 eV) at an ion-source temperature of 200°C. Mass spectra were obtained in full-scan mode from mass/charge ratio (m/z) 35 to 500. GCMSsolution software (version 4.2; Shimadzu Co.) was used to process the raw data, including peak picking from total ion chromatograms, peak-peak signal-noise (S/N) ratio calculation, and peak area measurement. Compounds were tentatively identified by searches of the Wiley MS library (seventh edition). RIs were determined using n-alkanes (C9–C40; GL Sciences, Tokyo, Japan).
Qualitative analysis of fractions 3 and 4 prepared in step 1 used a ZB-WAX column (30 m × 0.32 mm internal diameter, 0.5 μm film thickness; Phenomenex, Torrance, CA, USA). Five microliters of each sample was injected using a CTC-Combi-PAL autosampler (AMR Inc., Tokyo, Japan) in splitless mode. The GC was operated with helium carrier gas, column flow (2.56 ml/min), and 150°C injector temperature. The GC oven temperature was maintained at 40°C for 2 min and increased to 160°C at a rate of 5°C/min.
Qualitative analysis of fraction 3-3-2-3 prepared in step 4 and co-injection with synthesized cis-trans nepetalactol used an InertCap Pure-WAX GC column (60 m × 0.25 mm internal diameter, 0.25 μm film thickness; GL Sciences). One microliter of each sample was injected using an AOC-20i/s autosampler (Shimadzu Co.) in splitless mode. The GC was operated with helium carrier gas, column flow (1.5 ml/min), and 150°C injector temperature. The GC oven temperature was maintained at 40°C for 2 min, increased to 250°C at a rate of 4°C/min, and held at 250°C for 20 min. In the co-injection experiments, 1 μl of synthesized cis-trans nepetalactol solution (109 ng/μl) was spiked with 1 μl of fraction 3-3-2-3 prepared in step 4.
This GC/MS condition was also used to quantify nepetalactol, isoiridomyrmecin, iridomyrmecin, isodihydronepetalactone, and dihydronepetalactone in the extract of silver vine leaves. Nepetalactol (RI = 2080), isoiridomyrmecin (RI = 2170), iridomyrmecin (RI = 2156), isodihydronepetalactone (RI = 2109), and dihydronepetalactone (RI = 2196) were quantified on the basis of the areas of the m/z 135, 67, 95, 81, and 81 peaks, respectively, extracted from the full-scan data. Calibration curves for nepetalactol and other compounds were generated using 1.08, 2.16, 8.4, 34.2, 136.8, and 546.0 μg/ml and using 0.18, 0.36, 1.4, 5.7, 22.8, and 91.0 μg/ml, respectively.
Behavioral assays using cis-trans nepetalactol in felid and nonfelid species
Each stock solution (nepetalactone, nepetalactol, isoiridomyrmecin, iridomyrmecin, isodihydronepetalactone, and dihydronepetalactone) was prepared by dissolution in ethanol at a concentration of 5 μg/μl. Stock solutions (10 μl of aliquots) and 10 μl of ethanol (negative control) were pipetted onto the filter paper adhered to the bottom of separate petri dishes 10 min before the start of a behavioral assay, allowing the ethanol solvent to evaporate under atmospheric conditions before testing. Fifteen of the 18 positive responder laboratory cats were put into test cages (88 cm × 57 cm × 70 cm or 93 cm × 63 cm × 116 cm) at least 5 min before each assay to settle. Immediately before each assay, two covered petri dishes containing nepetalactol-impregnated filter paper (nepetalactol-paper) and control filter paper (control-paper) were fixed on the floor of the cage 30 cm apart using double-sided tape before removing the lids. Nepetalactol-paper was presented randomly on the right or left. Behavior was recorded using a digital video camera, until at least approximately 5 min after the cat showed disinterest in the dishes. The subject cats were not given treats (e.g., food or toys) 2 hours before or after the behavioral assays. The duration of face rubbing and rolling over was assessed using BORIS (50), which was also used to plot the rubbing and rolling activity patterns in response to nepetalactol- and control-papers in each cat. Twelve of the eighteen positive responder cats were also tested for responsiveness toward six iridoid compounds, as described above. Behavioral assays were carried out only once per day in each cat. The order of presentation of six compounds was determined randomly.
Behavioral assays using 30 feral cats were carried out at Tashirojima, a small island off the coast of mainland Japan known informally as “cat island,” which is inhabited by several hundred felines cared for and worshiped by the island’s human residents. When observers encountered a free-ranging feral cat, a nepetalactol (50 μg)–paper and a control-paper were presented in separate open clean petri dishes, placed on the ground approximately 1 m from the subject and 30 cm apart. The location of the nepetalactol-paper was randomized to the right or left of the control-paper. Behavior was recorded using a digital video camera until the cat became disinterested in the dishes and left. The duration of face rubbing and rolling over was assessed using BORIS (50).
To test other felids in zoos, thick filter papers (Advantec chromatography no. 526; Toyo Roshi Kaisha Ltd.) were cut to 8 cm × 6 cm and adhered to polyethylene terephthalate sheets (1 mm thickness; Acrysunday Co. Ltd., Tokyo, Japan) of the same size using double-sided tape. The stock solution of nepetalactol (400 μl of aliquots for jaguars and Amur leopard and 200 μl of aliquot for Eurasian lynx) and the same volume of ethanol were pipetted onto separate thick filter papers, allowing the solvent ethanol to evaporate under atmospheric conditions. These papers were adhered to the floor of the felids’ backyard areas with super-glued double-sided tape, and the subject felids were then allowed to enter their individual backyards. A video camera was used to record the behavior of each subject from 15 min up to 2.5 hours, until the animals showed disinterest in the papers. The duration of face rubbing and rolling over was assessed using BORIS software.
Behavioral assays of dogs were carried out in their familiar home environment to minimize any stress: five border collies in cages (114 cm × 79 cm × 65.5 cm) that they were familiar with, a mixed-breed dog tested outdoors near the front door of the owner’s house, and the other dogs inside the owner’s house. Each assay presented individual dogs with two petri dishes containing nepetalactol (200 μg) on paper versus control-paper, adhered to the floor 30 cm apart using double-sided tape.
To assay the response of laboratory mice, individual subjects were tested in cages (39 cm × 25 cm × 12 cm) with filter paper (Advantec chromatography no. 526) covering the floor, during the light period. A stock solution of nepetalactol (250 μg) was applied onto the filter paper in two locations and 50 μl of ethanol control in one location on the paper, each 22 cm apart. After the paper had air-dried, the mouse was transferred to the cage and video-recorded for 5 min.
Plasma β-endorphin concentration
On day 1, blood samples were taken from five cats 5 min before presentation of nepetalactol (200 μg)–paper or control-paper and again immediately after the feline response toward nepetalactol. On day 5, blood samples were taken from the same cats 5 min before the presentation of control-paper only and after the same duration as their response shown on day 1 (Fig. 3A). Blood (1 ml) was collected from the cephalic vein using a 23-gauge needle using heparin (Mochida Pharmaceutical Co. Ltd., Tokyo, Japan). All blood samples were centrifuged at 800g for 10 min, and the plasma was stored at −80°C until analysis (all completed within 2 months of sampling). As the amino acid sequence of domestic cat β-endorphin (YGGFMTSEKSQTPLVTLFKNAIIKNAHKKGQ) completely matches that of mouse β-endorphin, plasma β-endorphin levels were measured using a mouse β-endorphin ELISA kit (NBP2-78775; Novus Biologicals, Littleton, CO, USA) according to the manufacturer’s instructions.
Pharmacological inhibition of the μ-opioid system in domestic cats
Naloxone hydrochloride dihydrate (naloxone) was dissolved in saline to a final concentration of 2.4 mg/ml (corresponding to 2 mg/ml of naloxone) and stored at 4°C until use. Six positively responding subject cats (the minimum required for statistical analysis, see below) were used for behavioral assays in 88 cm × 57 cm × 70 cm cages (see schematic in Fig. 3C). On day 1, cats were administered a saline control treatment [0.1 ml/kg intramuscularly (IM)] into the quadriceps muscles using 1-ml syringes with 25-gauge needles. After 5 min, each cat was presented with nepetalactol (200 μg)– and control-papers in separate petri dishes, fixed to the cage floor 30 cm apart using double-sided tape. Behavior was video-recorded for approximately 10 min. On day 2, the cats were administered either naloxone (0.2 mg/kg IM) or a saline control treatment (0.1 ml/kg IM) into the quadriceps muscles using 1-ml syringes with 25-gauge needles, followed by presentation of stimuli in two dishes as on day 1. The duration of face rubbing and rolling over to nepetalactol-paper was measured 5 to 15 min following saline or naloxone administration, using BORIS software. In addition, to assess the effect of naloxone on more general activity, the duration of walking around the cage and grooming was calculated over the first 5 min of the test (this was measured over the first half of the test only because cats showing a behavioral response become relatively inactive for a short period afterward so would not be equivalent between treatments).
Experiments to examine behavioral function of silver vine response in cats
Seven positive responder laboratory cats were habituated to individual test cages (60 cm × 44 cm × 54 cm or 93 cm × 63 cm × 116 cm for high-ceiling cage tests) for at least 5 min before each test. Cats were presented with a choice between test versus control-papers presented in two separate petri dishes fixed either to opposite cage walls (20 cm above the floor) or on the ceiling, 30 cm apart. Dishes were attached to the cages using two elastic bands inserted into four 4-mm-diameter holes in the bottom of each petri dish. As in earlier tests, a filter paper (Advantec qualitative no. 1) was adhered to the bottom of each dish using double-sided tape. Nepetalactol stock solution (40 μl; 200 μg) or ethanol was pipetted onto filter papers, allowing the ethanol solvent to evaporate before lids were placed on the dishes (held on with gummed cloth tape). Petri dishes with and without nepetalactol were presented randomly on the right or left of the cage, and lids were removed immediately before the assay. Behavior was recorded with a digital video camera for approximately 10 min, until the cats showed disinterest in the dishes. The number of face rubbing events was counted using BORIS software.
To assess whether nepetalactol was transferred to the cat’s face and head fur during the behavioral response, donor cats were presented with two petri dishes containing nepetalactol (200 μg)–paper versus control-paper (Fig. 5D). The combination of donor and subject cats is shown in table S2. Dishes were either open (to allow contact) or covered by lids pierced with twenty 4-mm-diameter holes (preventing contact). Immediately after donor cats had completed a behavioral response to nepetalactol-paper, the face and head of the donor were wiped with a filter paper (Advantec qualitative no. 1) soaked with 600 μl of ethanol. The same areas of control donor cats that had not been presented with any chemical compound were similarly wiped. Once the ethanol had evaporated, subject cats were tested with a choice of wipes from a behavioral response versus control donor, as described above.
Mosquito repellence assays
Adult female A. albopictus mosquitoes (2 to 5 days since emergence) were purchased from Sumika Technoservice Corporation (Hyogo, Japan). We used only female A. albopictus because females, but not males, require mammalian blood for egg development (51). All assays were carried out using 3- to 9-day-old A. albopictus at 24°C and recorded using digital video cameras.
Mosquito repellent properties of nepetalactol and silver vine leaves were examined as described below. A. albopictus were placed into acrylic cages (20 cm × 20 cm × 20 cm) with 14 to 22 A. albopictus per cage. Cages had seven air vents (3 mm diameter), while a hole (10 cm diameter) in one side of the cage was connected to a plastic bag (26 cm depth) that acted as a shelter (Fig. 4A). Test stimuli consisted of nepetalactol in ethanol (50 μg, 200 μg, and 2 mg of nepetalactol), a solvent control (400 μl of ethanol), or five silver vine leaves, each presented in the base of a petri dish (6 cm diameter; AS ONE Corp., rubbed with 120-grit sandpaper). After solvent evaporation, the dishes were placed on the floor of separate mosquito cages. After 10 min, two observers counted the number of A. albopictus present in the plastic bag shelters. Mosquito repellency was measured as the percentage of mosquitoes in the plastic bag shelter. Each stimulant was tested four times in different cages.
To examine mosquito repellence from cat heads, 30 A. albopictus were placed into acrylic cages (40 cm × 20 cm × 20 cm) with 14 air vents (3 mm diameter) and two holes (10 cm diameter) in opposite sides of the cage that allowed the insertion of the heads of two anesthetized cats, with plastic bags (17 cm depth) around their necks to prevent mosquito escape. All assays were carried out using 3- to 7-day-old A. albopictus at 24°C and recorded using digital video cameras. Anesthesia of all cats was induced with medetomidine hydrochloride (0.15 mg/kg IM injection; Dorbene, Kyoritsuseiyaku Corp., Tokyo, Japan) into the quadriceps muscle using 1-ml syringes with 25-gauge needles. For ethical reasons, these tests used the minimum number of cats required to achieve statistical significance based on a predicted consistent avoidance of the same treatment in each trial and nonparametric two-tailed analysis (six pairs).
In the first experiment, a Kimwipe (Kimberly-Clark Corp., Roswell, GA, USA) soaked with 5 ml of solvent (control) or nepetalactol (500 μg) was used to wipe the fur and skin of each cat’s head and allowed to evaporate for 5 min. Control and nepetalactol-treated heads were inserted into the same cage of 30 A. albopictus, randomly assigned to the right and left sides of the cage. The number of A. albopictus landing on each head over 10 min was counted by two observers, one for each cat. Immediately after assays, the cats were recovered from anesthesia with atipamezole hydrochloride (0.15 mg/kg IM; Atipame, Kyoritsuseiyaku Corp.) injected into the quadriceps muscles using 1-ml syringes with 25-gauge needles.
In a second experiment, one cat in each pair that was a positive responder to silver vine was stimulated to show the characteristic behavioral response with approximately 50 g of fresh silver vine leaves (cages 93 cm × 63 cm × 116 cm). Immediately afterward, the cat was anesthetized with a paired unstimulated cat and their heads were placed into a test cage to count the number of A. albopictus landing on each head over 10 min as described above. As a control experiment, one cat in each pair that either was a negative responder to silver vine (n = 4) or had been administered naloxone (0.2 mg/kg IM) to inhibit a behavioral response (n = 2) was presented with the same amount of silver vine leaves for about 5 min. Immediately afterward, the cat was then paired with an unstimulated cat and used to assess the number of A. albopictus landing on each head over 10 min as described above.
Data analysis
Analyses were performed in SPSS (IBM version 25) or R (version 3.6.2, R Core Team 2014). Where parametric analyses were used, QQ normal plots and Shapiro-Wilks tests confirmed that residuals approximated normality. Tests were two-tailed unless otherwise indicated. All raw behavior data are provided in the Supplementary Materials.
More prolonged rubbing and rolling targeted to silver vine extract or nepetalactol were compared to the matched control stimulus using nonparametric Wilcoxon matched-pair signed ranks tests (exact P values calculated where n < 10). As we were only interested in what stimulated a positive response and the same conclusion of lack of response would be drawn whether cats showed an equal or more prolonged response to the control, we appropriately used directional one-tailed tests to assess this response. However, in each case, a two-tailed test would also have demonstrated a statistically significant difference between treatment and control (P < 0.05), except for the test of zoo felids where the number of animals that could be tested was strictly limited and reduced the statistical power of the test.
The duration of rubbing and rolling targeted to nepetalactone, nepetalactol, isoiridomyrmecin, iridomyrmecin, isodihydronepetalactone, and dihydronepetalactone was compared using a Friedman test, with differences between pairs of groups examined by Bonferroni-Dunn post-hoc comparisons. In experiments where there were only two treatment groups, Mann-Whitney U tests checked whether the bias in behavioral response duration (test stimulus − control) differed between treatments. The frequency of positive and negative responses shown by dogs or mice was compared to laboratory cats using Fisher’s exact tests. A contingency chi-square test assessed whether the occurrence of rolling behavior differed when test stimuli were presented on the test cage floor versus on the cage walls or ceiling.
Plasma β-endorphin concentrations were compared immediately before and after exposure to nepetalactol (day 1) or to a control stimulus (day 5) using a repeated-measures ANOVA. Separate analyses examined the change in β-endorphin level in behavioral response to each stimulus after finding a significant interaction between stimulus and sample time point. Wilcoxon matched-pair signed ranks test (two-tailed, as naloxone could have decreased or increased the behavioral response) compared the duration of rubbing and rolling response following saline administration (day 1) and naloxone or saline administration (day 2). Similar tests also examined the effect of naloxone on the duration of walking and grooming over the first 5 min of the test to check for any more generalized effects on activity.
To establish whether contact rubbing during the behavioral response transferred nepetalactol onto the head fur of the responding cat, the behavioral response of test subjects to a test paper used to wipe a donor cat immediately after the donor had shown a behavioral response to nepetalactol was compared to a test paper used to wipe an unstimulated cat. Subjects were given two tests with the same donors, when the donor cats could physically contact the nepetalactol-paper or not. We predicted that subjects would only show a substantial response toward wipes from donors that had been allowed direct contact with nepetalactol and not to wipes from donors exposed to nepetalactol that could not physically contact the stimulus or to wipes from unstimulated donor controls. For analysis, one donor in each trial (stimulated or unstimulated) was randomly assigned by coin toss as the focal donor, and the within-trial bias in the behavioral response calculated as the duration of rubbing and rolling on the focal donor wipe minus response to the nonfocal donor wipe. A linear mixed effects model [using the lme4 package in R (52)] examined the predicted interaction between the fixed effects of focal donor nepetalactol stimulation (yes or no) and whether the stimulated donor had physical contact with nepetalactol (yes or no) on the bias in the behavioral response shown by subjects, with subject cat as a random effect. Likelihood ratio tests compared the full model against a reduced model without the effect of interest using the “anova” function.
To examine repellence of A. albopictus, the percentage of A. albopictus found in a bag shelter after 10-min exposure to nepetalactol at different concentrations, silver vine leaves, or solvent control was compared using a univariate ANOVA, with planned comparison of each test stimulus with the control. To assess repellence from cat heads, repeated-measures ANOVA compared the number of A. albopictus landing on the heads of anaesthetized cats that had been treated with 500 μg of nepetalactol versus a solvent control. Another repeated-measures ANOVA compared the number of A. albopictus landing on cats that had rubbed on fresh silver vine leaves versus an unexposed cat or landing on cats that had not rubbed on the leaves versus an unexposed cat.