- Many plants contain phenolic glycosides that are toxic for insect herbivores
- •Whitefly carries a plant-derived phenolic glucoside malonyltransferase gene BtPMaT1
- •BtPMaT1 enables whiteflies to neutralize phenolic glycosides in host plants
- •Plant-mediated silencing of BtPMaT1 confers tomato full resistance to whiteflies
Summary
Plants protect themselves with a vast array of toxic secondary metabolites, yet most plants serve as food for insects. The evolutionary processes that allow herbivorous insects to resist plant defenses remain largely unknown. The whitefly Bemisia tabaci is a cosmopolitan, highly polyphagous agricultural pest that vectors several serious plant pathogenic viruses and is an excellent model to probe the molecular mechanisms involved in overcoming plant defenses. Here, we show that, through an exceptional horizontal gene transfer event, the whitefly has acquired the plant-derived phenolic glucoside malonyltransferase gene BtPMaT1. This gene enables whiteflies to neutralize phenolic glucosides. This was confirmed by genetically transforming tomato plants to produce small interfering RNAs that silence BtPMaT1, thus impairing the whiteflies’ detoxification ability. These findings reveal an evolutionary scenario whereby herbivores harness the genetic toolkit of their host plants to develop resistance to plant defenses and how this can be exploited for crop protection.
Graphical abstract

Keywords
- Bemisia tabaci
- horizontal gene transfer
- phenolic glucoside malonyltransferase
- tomato
- detoxification
- co-evolution
- insect-plant interaction
- plant secondary metabolite
- pest control
Introduction
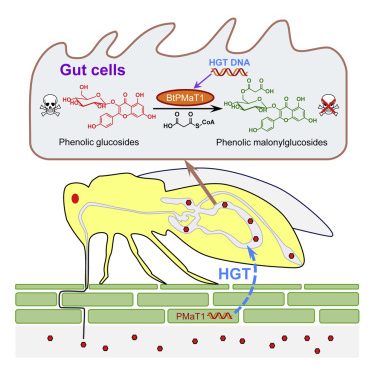
During more than 400 million years of co-evolution with insect herbivores (Price et al., 2011), plants have developed a highly diverse spectrum of morphological (Higuchi and Kawakita, 2019), molecular (Erb and Reymond, 2019), and biochemical (Mithöfer and Boland, 2012) defenses to withstand insect attack. Among the biochemical defenses, plant secondary metabolites are the most diverse and effective weapons that can be attributed to herbivore-plant co-evolution (Speed et al., 2015).Phenolic glycosides, which are comprised of a sugar unit bound to a phenol aglycone, are among the most abundant plant secondary metabolites. They strongly affect growth, development, and behavior of insect herbivores (Boeckler et al., 2011; Leiss et al., 2009; Mierziak et al., 2014; Onkokesung et al., 2014). In plants, an important modification of phenolic glycosides involves their malonylation, which is regulated by phenolic glucoside malonyltransferases (Taguchi et al., 2010). These enzymes can catalyze the transfer of a malonyl group from malonyl coenzyme A (CoA) to the phenolic glycosides and play an important role in various processes, including xenobiotic detoxification (Taguchi et al., 2005, Taguchi et al., 2010). Certain specialized insects, in particular those that feed on trees of the Salicaceae, can also readily cope with phenolic glycosides and may even use them as feeding and oviposition stimulants (Boeckler et al., 2011). They neutralize the defensive phenolic glycosides either by using specific detoxifying enzymes, decreasing the enzyme activity of β-glucosidases, directly sequestering phenolic glycosides, or converting them to salicylaldehyde (Boeckler et al., 2011; Després et al., 2007; Opitz and Müller, 2009). However, the mechanisms that allow generalist insects with a broad range of host plants to overcome the effects of phenolic glycosides are unknown.The sweet potato whitefly, Bemisia tabaci (Gennadius), is a species complex of at least 30 cryptic species, some of which (e.g., Mediterranean [MED] and Middle East-Asia Minor 1 [MEAM1]) are among the most devastating crop pests (De Barro et al., 2011; Liu et al., 2007). Outbreaks occur often worldwide, and B. tabaci can seriously reduce crop yields by feeding on phloem, transmitting plant viruses, and excreting honeydew (Gilbertson et al., 2015). It is extremely polyphagous—more than 600 host plant species have been recorded—and exhibits exceptional host adaptability (Oliveira et al., 2001). Most of its host plants contain phenolic glycosides, and uncovering how B. tabaci copes with these defense metabolites could help explain its pervasive host adaptability.Here, using bioinformatic, molecular, and biochemical approaches, combined with insect performance assays, we show that the B. tabaci genome harbors a plant-specific and horizontally transferred gene, BtPMaT1, encoding a phenolic glucoside malonyltransferase that enables the detoxification of phenolic glycosides. This discovery reveals an unexpected route by which B. tabaci has evolved its extraordinary ability to overcome the defenses of its host plants. Moreover, we show that interfering with the functioning of this plant-derived gene in B. tabaci can be a highly effective way to control this extremely important global pest.
Results
Identification and characterization of malonyltransferase genes in B. tabaci
Based on a preliminary survey of B. tabaci MED genes related to KEGG (Kyoto Encyclopedia of Genes and Genomes) pathways, we identified the BtPMaT1 gene (BTA023005.1). This gene consists of one exon (Figure 1A) and was cloned from B. tabaci MED adults using specific PCR primers (Table S1). The coding sequence (CDS) of the BtPMaT1 gene (GenBank: MN756010) spans 1,386 nucleotides and encodes a protein of 461 amino acids that lacks an N-terminal signaling peptide (Figure S1A). Compared with BTA023005.1, the cloned BtPMaT1 CDS contained several non-synonymous single-nucleotide polymorphisms (Figure S1B). A BLASTp search against the predicted protein dataset of the B. tabaci MED genome (http://gigadb.org/dataset/100286) (Xie et al., 2017) revealed the presence of BTA005164.2 (BtPMaT2), a close homolog of BtPMaT1 (BLASTp E-value of 7.9E−107). Both B. tabaci MED proteins carried the conserved HXXXD and DFGWG motifs of plant BAHD acyltransferases (named according to the first letter of each of the first four biochemically characterized enzymes of this family [BEAT, AHCT, HCBT and DAT]) (D’Auria, 2006). The YXGNC motif, typical for phenolic glycoside and anthocyanin malonyltransferases (belonging to “clade I” BAHD acyltransferases) (D’Auria, 2006; Tuominen et al., 2011; Zhao et al., 2011), was also present (Figure S2). In this study, we primarily investigated the potential role of BtPMaT1 in the detoxification of phenolic glycosides, and whether BtPMaT2 also plays a potential role in neutralizing plant metabolites remains to be determined.



Evidence for the horizontal transfer of BtPMaT1 from plants to whiteflies
BLASTp and tBLASTn searches against the NCBI non-redundant database revealed that BtPMaT1 had its closest homologs in plants (E-value < E−90, bit-score > 300) and did not have homologs in other arthropods, except for other B. tabaci cryptic species (e.g., XP_018897056.1/Bta07786 in B. tabaci MEAM1) (Figure 1D; Figure S3). An InterProScan search (Jones et al., 2014) of BtPMaT1 revealed the presence (E-value of 1.7E−110) of the PANTHER domain PTHR31625, which has to date only been detected in plant and fungal proteins (3,073 sequences) and one bacterial protein (GenBank: KPK61911) (see “Taxonomy” tab at https://www.ebi.ac.uk/interpro/entry/panther/PTHR31625/, accessed on November 15, 2020). Last, a maximum likelihood phylogenetic analysis showed that BtPMaT1 clustered within a group of plant BAHD acyltransferases containing the PTHR31625 domain and the YXGNC motif (Figure 1B, Figure S4; Data S1).


Independent genomic analyses were then performed to confirm that BtPMaT1 is indeed integrated into the B. tabaci MED genome. We found that BtPMaT1 is located on scaffold 523 surrounded by two typical arthropod serine protease genes (BTA023004.3 and BTA023006.1), both of which contains 9 exons (Figure 1D). Identical neighboring genes were also found for BtPMaT1 in the B. tabaci MEAM1 genome (Figure 1D), whereas no ortholog of BtPMaT1 was found in the region near a gene of the greenhouse whitefly, Trialeurodes vaporariorum (Tv04085 on scaffold 2), that was the most homologous to the two flanking B. tabaci serine proteases genes (both XP_018897238.1/Bta07787 and XP_018897055.1/Bta07785 of B. tabaci MEAM1 had their best BLASTp hit with Tv04085 [E-value of E−163 and E−58, respectively]). It was also not found in any other region of the T. vaporarorium genome. PCR confirmed that BtPMaT1 was integrated between the two serine protease genes in the B. tabaci MED genome (Figure 1C). Despite an uneven coverage of New World B. tabaci, the coverage was highly consistent in the BtPMaT1 gene region among different B. tabaci cryptic species (Figure 1A). Finally, the expression of BtPMaT1 was detected in all strains of B. tabaci MED that were reared on different host plants (Figure 1E), indicating the presence of BtPMaT1 is independent of B. tabaci strain and host plant. Overall, our analyses show that the BtPMaT1 gene is not a plant gene contaminant and strongly indicate that BtPMaT1 has been horizontally acquired from plants.
The spatio-temporal expression profiling of the BtPMaT1 gene
The spatio-temporal expression pattern of BtPMaT1 was monitored with real-time quantitative PCR (qPCR) using five developmental stages (eggs, 1st–2nd, 3rd, and 4th instar nymphs, adults) and various parts of the adults (head, thorax, abdomen, non-gut tissue, and gut tissue) of B. tabaci MED. This showed that BtPMaT1 was expressed in all developmental stages and tissues (Figures S5A–S5C). The significantly higher expression in adults suggests that BtPMaT1 plays its most important role in the adult stage. Moreover, BtPMaT1 was most highly expressed in the abdomen and especially in the gut, which indicates that it is a gut-specific gene that plays a key role in detoxification processes. Remarkably, BtPMaT1 expression was, albeit to a much lesser extent, also detected in the egg stage, providing additional evidence that the BtPMaT1 gene is not a plant gene contaminant.

Phenolic glycosides in tomato leaves and effect of phenolic glycosides on B. tabaci performance
To test whether the BtPMaT1 gene is indeed involved in the metabolism of phenolic glycoside in host plants, we first determined the classes of phenolic glycosides in tomato leaves using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS). The experimental process as shown in Figure 2A resulted in UV and MS spectra (Figures 2B and 2C) that detected a total of 9,873 metabolites, and 6,005 compounds were identified by Progenesis QI analysis. Among them, 290 were identified as phenolic glycosides by manual filtering (Figure 2D; Table S2). Following a previous study (Lattanzio et al., 2006), the phenolic glycosides were assigned to 9 categories based on their basic skeleton dominated by C6–C3–C6 glycosides (Figure 2E; Table S2). Subsequently, 11 representative phenolic glycosides from each category, present in a variety of different plant species, were selected (Figure 2; Table S2) to explore their possible impact on the performance of B. tabaci adults. Bioassay results demonstrated that the adult mortality caused by five of the phenolic glycosides (kaempferol 3-O-glucoside, kaempferol 7-O-glucoside, phenyl β-D-glucoside, phlorizin, and rhaponticin) was significantly higher than the control, whereas mortality caused by the remaining six compounds (4-nitrophenyl β-D-glucoside, salicin, androsin, verbascoside, 4-methylumbelliferone glucoside, and polygalaxanthone III) was not significantly different from the control (Figure 3B), implying that several phenolic glycosides are toxic to B. tabaci when ingested at a dose (10 μM) that is higher than what they are normally exposed to when feeding on plant phloem.


Functional analysis of the BtPMaT1 gene using RNAi and VIGS assays
To validate the role of BtPMaT1 in phenolic glycoside detoxification, we performed dietary RNA interference (RNAi) by directly feeding its specific double-stranded RNA (dsRNA) (dsBtPMaT1) to B. tabaci MED adults (Figure 3A). qPCR analysis at 48 h post-RNAi showed that BtPMaT1 expression was reduced by 49.2% compared with the control (Figure 3C). Silencing BtPMaT1 gene had no direct effect on B. tabaci survival (Figure 3D). Subsequent feeding assays revealed that silencing of BtPMaT1 significantly increased the mortality of adults feeding on kaempferol 3-O-glucoside (Figure 3E), kaempferol 7-O-glucoside (Figure 3F), or rhaponticin (Figure 3G), but did not significantly increase the mortality of adults feeding on phenyl β-D-glucoside (Figure 3H) or phlorizin (Figure 3I). This result confirms that BtPMaT1 plays a pivotal role in the detoxification of some, but not all, detrimental phenolic glycosides.Subsequently, we further tested the function of BtPMaT1 in an ecologically relevant experiment, with whitefly adults actually feeding on plants and using a virus-induced gene silencing (VIGS) technique. We first cloned specific gene-silencing fragments into the pTRV2 vector to construct the VIGS vectors pTRV2-BtPMaT1 and pTRV2-EGFP (Figure 3J). pTRV1 and recombinant pTRV2-BtPMaT1 and pTRV2-EGFP were then introduced into Agrobacterium tumefaciens. Equal volumes of A. tumefaciens containing pTRV1 and pTRV2 harboring the target gene were mixed and infiltrated into two true leaves of tomato plants using a needleless syringe (Figure 3K). After 20 days, silencing fragments of the BtPMaT1 gene and the EGFP gene were detected in the tomato seedlings by PCR (Figures 3L and 3M). Next, BtPMaT1 expression in B. tabaci adults that had been feeding on the tomato seedlings for 7 days was assessed by qPCR. Relative to expression in adults feeding on pTRV2-EGFP tomato plants, BtPMaT1 expression in adults feeding on pTRV2-BtPMaT1 tomato plants was reduced by 63.8% (Figure 3N). Moreover, continuous silencing of BtPMaT1 by VIGS for 7 days significantly increased mortality and reduced fecundity of B. tabaci adults (Figures 3O and 3P).
The BtPMaT1 protein metabolically detoxifies phenolic glycosides
To confirm that BtPMaT1 is indeed able to acylate phenolic glycosides, BtPMaT1 was heterologously expressed in Spodoptera frugiperda (Sf9) cells (Figure S6) for in vitro enzyme activity tests. UPLC-QTOF/MS analyses showed that the recombinant BtPMaT1 protein has malonyltransferase activity against three of the eleven tested phenolic glycosides: kaempferol 3-O-glucoside, kaempferol 7-O-glucoside, and rhaponticin. Each was converted into their respective malonylglucoside (Figure 4), with a higher activity to the flavonoid substances kaempferol 7-O-glucoside and kaempferol 3-O-glucoside than to the non-flavonoid rhaponticin (Figure 5B). Flavonoids are very abundant in plants, in particular kaempferol, quercetin, apigenin, and naringenin, which mainly exist in a glycosylated state. Based on our results, we hypothesize that BtPMaT1 is able to malonylate flavonoids other than kaempferol glucoside, which we confirmed for isoquercetin (quercetin 3-O-β-D-glucopyranoside), apigetrin (apigenin-7-O-glucoside), and prunin (naringenin-7-O-glucoside) (Figures 4 and 5B). Moreover, we also confirmed the acylation activity of BtPMaT1 in vivo, observing that kaempferol 3-O-glucoside, kaempferol 7-O-glucoside, rhaponticin, isoquercetin, apigetrin, or prunin were metabolized when they were incubated with the dissected and pooled B. tabaci adult gut tissues (Figure 5C). This further confirms the expected metabolic function of the BtPMaT1 protein and suggests that the malonylation reaction occurs inside the B. tabaci adult gut tissues.



To further test whether BtPMaT1 can in vivo metabolize phenolic glycosides in B. tabaci, we collected the honeydew after oral feeding of a representative phenolic glycoside and after feeding on transgenic tomatoes (Figure 5A). After B. tabaci adults were fed a representative phenolic glycoside (kaempferol 3-O-glucoside) for 48 h, kaempferol 3-O-glucoside and malonylated kaempferol 3-O-glucoside were found in the honeydew (Figures 5E and 5F). Moreover, silencing of BtPMaT1 by oral delivery of its specific dsRNA significantly increased kaempferol 3-O-glucoside and decreased malonylated kaempferol 3-O-glucoside in honeydew (Figures 5G and 5H), supporting the notion that BtPMaT1 catalyzes malonylation of phenolic glycosides in vivo in B. tabaci. The role of malonylation of phenolic glycosides catalyzed by the BtPMaT1 protein in a metabolic detoxification process was further validated by feeding B. tabaci adults on diet solution with kaempferol 3-O-glucoside and the corresponding phenolic malonylglycoside
[kaempferol 3-O-(6-malonyl-glucoside)]
(Figure 5D). Mortality of B. tabaci adults fed on kaempferol 3-O-glucoside was significantly higher than those fed on control diet. By contrast, the kaempferol 3-O-(6-malonyl-glucoside) diet had a far less lethal effect, further confirming the important role of malonylation catalyzed by BtPMaT1 in the detoxification of phenolic glucosides. We also performed a whole-metabolome assay of the honeydew of B. tabaci fed on wild-type and transformed tomato plants that silence the BtPMaT1 gene, as described in the next section. As shown in Figure 5I and Tables S3 and S4, a total of 2,966 metabolites were detected in the whitefly honeydew, and 94 flavonoid glycosides and 4 flavonoid malonylglucoside compounds could be identified. Among them, the relative levels of 50 flavonoid glycosides were significantly higher in the honeydew of B. tabaci fed on transformed tomato plants, while the four detected flavonoid malonylglucosides were significantly lower, supporting the phenolic glucoside malonyltransferase function of the BtPMaT1 gene.
The transgenic expression of dsBtPMaT1 in tomato enhances resistance to whiteflies
A final experiment was conducted to validate the notion that impairing BtPMaT1 in B. tabaci can enhance plant resistance to the pest. Hairpin RNA of BtPMaT1 was produced by transcription of two inverted repeats of 444-bp target fragment of BtPMaT1. The transfer DNA (T-DNA) region of the hairpin RNA expression vector (pCAMBIA-RNAi-BtPMaT1) as shown in Figure S7A was transferred to tomato using A. tumefaciens-based genetic transformation (Figures S7B–S7F). Positive transgenic lines were identified by PCR amplification (Figure 6A). Northern blot analyses confirmed that positive transgenic lines contained both long dsRNAs and small interfering RNAs (siRNAs) of BtPMaT1 (Figure 6B). Gene-silencing efficiency in whitefly adults was confirmed by qPCR and showed that 7 days of feeding on transgenic tomatoes significantly reduced BtPMaT1 expression compared with the control (Figure 6C). Mortality of whiteflies was already higher after 1 day of feeding on transgenic tomatoes and increased over time (Figure 6D). Most importantly, in field simulation cages, feeding on transgenic tomatoes results in almost 100% mortality of whiteflies compared with less than 20% in the control (Figures 6E, 6J, and 6K). By contrast, the performance of a non-target insect, the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae), was unaffected on transgenic plants after 7 days of feeding in both clip cages (Figure 6F) and field simulation cages (Figures 6G, 6L, and 6M). Likewise, the mortality of another non-target arthropod pest, the spider mite Tetranychus urticae (Acarina: Tetranychidae) was unaffected after 7 days of feeding on transgenic plants either in individual leaf assays in Petri dishes (Figure 6H) or on whole plants (Figures 6I, 6N, and 6O).


Discussion
The ability of herbivorous insects to detoxify plant defense compounds is pivotal for their adaptive evolution (Heidel-Fischer and Vogel, 2015), and it is increasingly evident that the modification of secondary metabolites by detoxifying enzymes is a highly effective strategy to neutralize plant toxins, also applied by whiteflies (Malka et al., 2020). The above-mentioned experiments reveal an uncommon evolutionary route by which whiteflies have gained the ability to malonylate a common group of plant defense compounds through the acquisition of a plant detoxification gene. The horizontal transfer of BtPMaT1 is shown to empower whiteflies with the ability to attach a malonyl group to phenolic glucosides, rendering these common plant-produced secondary metabolites almost completely innocuous (Figure 7). The metabolic detoxification process in B. tabaci most likely relies on a conjugation reaction similar to herbicide metabolism in certain plants, whereby malonyltransferase can catalyze detoxification by conjugating the herbicide or an intermediate metabolite with malonyl-CoA (Frear et al., 1983; Lao et al., 2003). Moreover, BtPMaT1 malonylation might promote the solubility and export of the ingested phenolic glycosides (Taguchi et al., 2010; Zhao, 2015). BtPMaT1 malonylation might also prevent glucosidase action on phenolic glycosides, as previously shown for malonylation of anthocyanins (Suzuki et al., 2002), and hence, reminiscent of adaptation mechanisms reported for insects feeding on Salicaceae, possibly limits the release of the (more) toxic aglycones (Ahmad et al., 1986; Lindroth, 1988). Intriguingly, our phylogenetic analyses showed that BtPMaT1 not only clustered with several plant phenolic glucoside malonyltransferases (AtPMaT1, AtPMaT2, Vh3MaT1, Lp3MaT1, and NtMaT1) but also with isoflavone glucoside (GmIMaT3, GmMaT4, and MtMaT4) and anthocyanin (Ss5MaT1) malonyltransferases (Figure S4), implying that additional substrates might be malonylated by BtPMaT1. Together with the presence of BtPMaT2 (Figure 1B; Figure S4), this likely broadens the spectrum of defensive compounds that can be neutralized by B. tabaci, warranting further study.

The heterologous expression of BtPMaT1 protein in Sf9 cells was found to catalyze the malonylation of phenolic glucosides (especially flavonoid glucosides). The malonylated product was detected in the honeydew after B. tabaci fed on kaempherol 3-O-glucoside, while suppressing the BtPMaT1 gene expression significantly decreased levels of this glucoside in the honeydew of B. tabaci. Moreover, we detected specific BtPMaT1 expression and malonyltransferase activity of BtPMaT1 inside the gut tissue. The enzyme possesses no signal peptide and must mainly exert its function in the cytosol of the gut cells rather than in the gut lumen. Hence, flavonoid glucosides need to enter the gut cells first, which might require a similar transport mechanism as in mammals. In mammals, one type of flavonoid glucoside can be directly transported into the gut cells by the sodium-dependent glucose transporter 1 (SGLT1) located on the cell membrane (Walle, 2004). The other group of flavonoid glucosides first needs to be hydrolyzed into flavonoids in the gut lumen and then absorbed into gut cells by passive diffusion (Terahara, 2015). UDP-glucuronosyltransferases (UGTs) are known to catalyze glucuronic acid conjugation of flavonoids to produce flavonoid glucosides in mammalian gut cells (Walle, 2004). Considering that in total 76 UGTs were identified in the B. tabaci MED genome (Guo et al., 2020), we can safely speculate that these whitefly UGTs catalyze the glycosylation of flavonoids with UDP-glucose to generate flavonoid glucosides in B. tabaci gut cells as previously reported for T. urticae UGTs (Snoeck et al., 2019). Subsequently, these phenolic glucosides can be malonylated in the gut cells by BtPMaT1 via the malonylation reaction as described above. It should be noted that more that 40% of the UGTs do not have a signal peptide (Guo et al., 2020) and are probably cytosolic UGTs that act in concert with BtPMaT1. The flux of malonylated flavonoid glycoside back into the gut lumen might then occur via ATP-binding cassette (ABC) transporters, similar to what has been reported for transport of flavonoids in plants (Zhao, 2015). Overall, our findings reveal that the malonylation of phenolic glycosides catalyzed by BtPMaT1 in gut cells is a key detoxification process in whiteflies.The evolutionary significance of horizontal gene transfer (HGT) is widely recognized for prokaryotes, but it is now becoming evident that it is also an important driving force for the adaptive evolution of eukaryotes (Husnik and McCutcheon, 2018). Novel traits that evolve through HGT can lead to the exploitation of new resources and niches (Bublitz et al., 2019; Kominek et al., 2019; Soucy et al., 2015; Wang et al., 2020; Wybouw et al., 2016). The donors in HGT events known for arthropods are almost exclusively microorganisms (Wybouw et al., 2016), and empirical evidence for horizontal transfer of functional plant-derived genes to insects has been missing. We show that plant-derived BtPMaT1 has retained its function and aids B. tabaci in coping with ingested phenolic glycosides, present in many of its host plants. Notably, horizontally transferred BtPMaT1 was detected in different cryptic species of B. tabaci, while it was not found in genomes of other insects, including the related greenhouse whitefly, T. vaporariorum (Xie et al., 2020). Bemisia must have acquired this gene after the divergence from Trialeurodes (~86 mya) (Santos-Garcia et al., 2015), and one could hypothesize, that (in addition to other factors) this might explain why Bemisia performs better on tomato compared with Trialeurodes (Zhang and Wan, 2012).The HGT of BtPMaT1 allows the whitefly to use a key element of the plants’ arsenal to protect itself from plant defense metabolites (Figure 7). Indeed, tomato also has a phenolic glucoside malonyltransferase (GenBank: XP_025883531, a homolog [68% identity] of NtMaT1 [Taguchi et al., 2005]). This is reminiscent of Han Feizi, a text written during the 3rd century BC by political philosopher Han Fei, in which he famously tells the story of “Attack your shield with your spear.” Similar to the gist of this story, we reveal here that whiteflies have adopted their opponent’s combat strategy to resist it, and we show that we can apply this same philosophy to control whiteflies. A good understanding of the molecular basis of B. tabaci’s polyvalent ability to overcome plant defenses is recognized as a key for the development of durable pest control strategies (Heidel-Fischer and Vogel, 2015). Recent progress has been made. For instance, whiteflies were found to suppress plant defenses by leveraging the crosstalk between plant defense hormones (Zhang et al., 2013), and they may even do so in neighboring plants through the induction of specific volatile signals (Zhang et al., 2019). Several whitefly salivary proteins have been identified as effectors that are involved in this apparent manipulation of plant defenses by eliciting the salicylic acid-signaling pathway (Wang et al., 2019; Xu et al., 2019). Here, we show that whiteflies, in addition to suppressing the synthesis of defensive plant secondary metabolites, also have attained the ability to detoxify defense compounds by acquiring a gene from plants. By silencing BtPMaT1 in B. tabaci adults using different RNAi approaches, we showed that we can disable their immunity to phenolic glycosides. This greatly reduced whitefly performance and offers prospects for whitefly control strategies.Overall, our findings imply that B. tabaci is prone to accept horizontal transfer genes and that this may help to circumvent resistance traits of host plants. This insight into a co-evolutionary process that facilitates host plant adaptation in insects also reveals that interfering with laterally transferred genes can be a highly effective way to combat pests. Indeed, the transgenic expression of dsBtPMaT1 in tomato is lethal to the whiteflies, but does not affect non-target organisms nor does it affect the homologous gene in the host plant because of low nucleic acid sequence similarity. Mining for other functional horizontal transfer genes from host plants into insect pests should yield other key genes that can be excellent targets for the promising RNAi-based insect pest control strategy.
Limitations of study
This study provides conclusive evidence that the plant-derived gene BtPMaT1 allows whiteflies to detoxify plant phenolic glucosides, but additional work is needed to fully elucidate its possible other functions. As our phylogenetic analysis showed that BtPMaT1 not only clustered with plant phenolic glucoside malonyltransferases but also with an anthocyanin malonyltransferase, the BtPMaT1 detoxification potential might be broader than we show here. Similarly, we will still need to determine the specific function of the homolog BtPMaT2, which is likely to also contribute to the whitefly’s ability to neutralize plant defense metabolites. Also, although we showed that BtPMaT1 and BtPMaT2 were horizontally acquired from plants, we were not able to precisely identify the plant donor species, which might be unveiled when more plant genomes become available.Transgenic tomatoes expressing the hairpin RNA of BtPMaT1 gene caused very high whitefly mortality in semi-field experiments, suggesting that these plants may offer an immediate and highly effective solution to control the pest. This remains to be tested under realistic field conditions. The transformation has no apparent effects on the phenotype of young tomato plants, but we will still have to establish possible effects on more mature plants and on tomato yield. Before the transgenic plants can be commercialized, it also still needs to be determined whether the dsRNA produced by the plants affects the performance of other organisms. In this study, we already show that it has no significant effects on aphids or mites.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-His(C-term)-HRP Antibody | Thermo Fisher Scientific | Cat# R931-25; RRID: AB_2556554 |
| Goat anti-Mouse IgG H&L (HRP) secondary antibody | Abcam | Cat# ab7068; RRID: AB_955413 |
| Bacterial and virus strains | ||
| Agrobacterium tumefaciens GV3101 | This study | N/A |
| Agrobacterium tumefaciens LBA4404 | This study | N/A |
| Biological samples | ||
| DNA of whitefly and tomato samples | This study | N/A |
| RNA of whitefly and tomato samples | This study | N/A |
| Protein of whitefly gut tissues | This study | N/A |
| Whitefly honeydew and tomato leaves for metabolic profiling | This study | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Phenyl β-D-glucoside | Yuanye Biotech | Cat# S24698 |
| 4-nitrophenyl β-D-glucoside | Yuanye Biotech | Cat# S10138 |
| Salicin | MEC | Cat# HY-N0149 |
| Androsin | MREDA | Cat# M043612 |
| Verbascoside | MEC | Cat# HY-N0021 |
| 4-methylumbelliferone glucoside | Yuanye Biotech | Cat# S11118 |
| Polygalaxanthone III | Solarbio | Cat# SP8440 |
| Rhaponticin | MREDA | Cat# M044379 |
| Kaempferol 7-O-glucoside | Yuanye Biotech | Cat# B21130 |
| Kaempferol 3-O-glucoside | Yuanye Biotech | Cat# B21704 |
| Phlorizin | Yuanye Biotech | Cat# B20449 |
| Malonyl-CoA | Sigma-Aldrich | Cat# M4263 |
| Kaempferol 3-O-(6-malonyl-glucoside) | BOC Sciences | Cat# 81149-02-2 |
| Quercetin 3-O-β-D-glucoside | Meilunbio | Cat# MB7017 |
| Apigenin-7-O-glucoside | Psaitong | Cat# A10718 |
| Naringenin-7-O-glucoside | Psaitong | Cat# N10139 |
| Peonidin-3-glucoside | Sigma-Aldrich | Cat# 40796 |
| Acetonitrile | Thermo Fisher Scientific | Cat# A955-4 |
| Methanol | Thermo Fisher Scientific | Cat# A456-4 |
| Formic acid | Thermo Fisher Scientific | Cat# A117-50 |
| XbaI | NEB | Cat# R0145V |
| SacI | NEB | Cat# R3156V |
| BamHI | NEB | Cat# R0136V |
| HindIII | NEB | Cat# R0104V |
| XhoI | NEB | Cat# R0146M |
| BglII | NEB | Cat# R0144L |
| T4 DNA Ligase | NEB | Cat# M0202L |
| Protease inhibitor cocktail (Complete tablets EDTA-free, EASYpack) | Roche | Cat# 4693132001 |
| DIG-11-UTP | Roche | Cat# 03359247910 |
| Critical commercial assays | ||
| SuperSignal West Pico Chemiluminescent Substrate | Thermo Fisher Scientific | Cat# 34577 |
| PrimeScript II 1st strand cDNA synthesis kit | TaKaRa | Cat# 6210B |
| PrimeScript RT kit | TaKaRa | Cat# RR014A |
| pEASY-T1 vector | TransGen | Cat# CT101-01 |
| Trans1-T1 competent cells | TransGen | Cat# CD501-02 |
| TIANamp Blood/Tissue/Cell Genomic DNA Extraction Kit DP304 | TIANGEN | Cat# DP304-02 |
| SuperReal PreMix Plus (SYBR Green) | TIANGEN | Cat# FP205-01 |
| 50 × ROX Reference Dye | TIANGEN | Cat# FP205-01 |
| T7 Ribomax Express RNAi System | Promega | Cat# P1700 |
| Bac-to-Bac Baculovirus Expression System | Invitrogen | Cat# 10359016 |
| Cellfectin II Reagent | Invitrogen | Cat# 10362100 |
| Sf-900 II SFM | Thermo Fisher Scientific | Cat# 21012026 |
| HisTrap HP 5 ml column | GE Healthcare | Cat# 17524802 |
| HiTrap desalting 5 ml column | GE Healthcare | Cat# 17140801 |
| Plant Genomic DNA Kit | TIANGEN | Cat# DP305-02 |
| Deposited data | ||
| Bemisia tabaci MED genome | Xie et al., 2017 | http://gigadb.org/dataset/100286 |
| RNA-seq data for Bemisia tabaci MED | Tian et al., 2017 | NCBI SRA: SRP064690 |
| Bemisia tabaci MEAM1 genome | Chen et al., 2016 | http://www.whiteflygenomics.org/cgi-bin/bta/index.cgi |
| Trialeurodes vaporariorum genome | Xie et al., 2020 | https://www.ncbi.nlm.nih.gov/assembly/GCA_011764245.1 |
| The BtPMaT1 gene of B. tabaci MED | This study | NCBI GenBank: MN756010 |
| The BtPMaT1 gene of B. tabaci New World | This study | NCBI GenBank: MW387250 |
| The BtPMaT1 gene of B. tabaci Asia II 3 | This study | NCBI GenBank: MW387251 |
| Experimental models: cell lines | ||
| Spodoptera frugiperda: cell line Sf9 | Invitrogen | Cat# B82501 |
| Experimental models: organisms/strains | ||
| B. tabaci MED reared on poinsettia (Euphorbia pulcherrima Wild. ex Kl.) | This study | N/A |
| B. tabaci MED reared on cotton (Gossypium herbaceum L. cv. DP99B) | This study | N/A |
| B. tabaci MED reared on tomato (Solanum lycopersicum Miller, cv. Zhongza 9) | This study | N/A |
| Myzus persicae reared on tomato (Solanum lycopersicum Miller, cv. Zhongza 9) | This study | N/A |
| Tetranychus urticae reared on tomato (Solanum lycopersicum Miller, cv. Zhongza 9) | This study | N/A |
| Tomato (Solanum lycopersicum Miller, cv. Zhongza 9) | This study | N/A |
| Tomato (Solanum lycopersicum cv. Moneymaker) | This study | N/A |
| Oligonucleotides | ||
| Primers for BtPMaT1 gene cloning, see Table S1 | This study | N/A |
| Primers for genome fragment cloning, see Table S1 | This study | N/A |
| Primers for RT-qPCR, see Table S1 | This study | N/A |
| Primers for RNAi, see Table S1 | This study | N/A |
| Primers for VIGS, see Table S1 | This study | N/A |
| Primers for BtPMaT1 heterologous expression, see Table S1 | This study | N/A |
| Primers for transgenic detection, see Table S1 | This study | N/A |
| Recombinant DNA | ||
| VIGS (pTRV1 and pTRV2) | Liu et al., 2002 | N/A |
| pTRV2-BtPMaT1 | This study | N/A |
| pTRV2-EGFP | This study | N/A |
| pRNAi1017 and PCAMBIA2300-35S-OCS | Xiong et al., 2013 | N/A |
| pRNAi-BtPMaT1 | This study | N/A |
| pCAMBIA-RNAi-BtPMaT1 | This study | N/A |
| Software and algorithms | ||
| InterProScan | Jones et al., 2014 | https://www.ebi.ac.uk/interpro/ |
| CD-HIT | Li and Godzik, 2006 | http://weizhongli-lab.org/cd-hit/ |
| MAFFT version 7 | Katoh and Standley, 2013 | https://mafft.cbrc.jp/alignment/software/ |
| CIPRES Science Gateway | Miller et al., 2012 | http://www.phylo.org/ |
| RaXML-HPC2 version 8.2.12 | Stamatakis, 2014 | https://github.com/stamatak/standard-RAxML |
| MEGA7 | Kumar et al., 2016 | https://www.megasoftware.net |
| Primer Premier 5.0 | Premier Biosoft | http://www.premierbiosoft.com/primerdesign/ |
| Progenesis QI | Waters | https://www.waters.com/waters/en_US/Progenesis-QI/nav.htm?cid=134790652&lset=1&locale=en_US&changedCountry=Y |
| UNIFI | Waters | https://www.waters.com/waters/en_US/Natural-Products-Application-Solution-with-UNIFI/nav.htm?cid=134777097&lset=1&locale=en_US&changedCountry=Y |
| IGV 2.3 | Broad Institute and the Regents of the University of California | http://software.broadinstitute.org/software/igv/ |
| SnapDragon | Harvard Medical College | https://www.flyrnai.org/cgi-bin/RNAi_find_primers.pl |
| GraphPad Prism 8 | GraphPad Software | https://www.graphpad.com/ |
| SigmaPlot 14.0 | Systat Software, Inc | https://systatsoftware.com/products/sigmaplot/ |
| SPSS V23.0 | IBM | https://www.ibm.com/analytics/spss-statistics-software |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Youjun Zhang (zhangyoujun@caas.cn).
Materials availability
The study did not generate new unique reagents.
Data and code availability
The full-length cDNA sequences of BtPMaT1 in B. tabaci MED, New World and Asia II 3 cryptic species of this study have been deposited in GenBank with the accession numbers MN756010, MW387250 and MW387251.
Experimental model and subject details
Arthropod strains
A strain of B. tabaci MED that was originally collected in 2009 from poinsettia (Euphorbia pulcherrima Wild. ex Kl.) in Beijing, was subsequently maintained on poinsettia and cotton plants (Gossypium herbaceum L. cv. DP99B) to establish a poinsettia strain and a cotton strain, respectively. A pepper strain that originated from B. tabaci MED collected from cucumber plants in Beijing in 2011, was subsequently transferred to caged pepper plants as previously described (Xie et al., 2017). A tomato strain of B. tabaci MED was collected in 2018 from tomato plants in Beijing and was subsequently reared on tomato (Solanum lycopersicum Miller, cv. Zhongza 9). The purity of each of these four strains was monitored by sequencing a fragment of the mitochondrial cytochrome oxidase I (mtCOI) gene every three to five generations (Chu et al., 2010). All of the B. tabaci strains were maintained in a glasshouse at 27 ± 1°C, 60%–80% relative humidity (RH), and a photoperiod of L16:D8. In this study, all four B. tabaci MED host plant strains were used to detect the presence of the BtPMaT1 gene while in all the other experiments only the cotton strain was used.The aphid Myzus persicae used in this study was originally collected from pakchoi in 2017 at Ningde, Fujian Province, China. Subsequently, this M. persicae population was maintained in the laboratory on radish seedling. A subset of this strain was transferred and continuously reared on tomato (Solanum lycopersicum Miller, cv. Zhongza 9) for more than one year at 26 ± 2°C, with a 60%–80% RH and a photoperiod of L16:D8, and was used in our experiments.The two-spotted spider mite T. urticae used in this study was originally collected from an apple orchard in 2009 at Tai’an, Shandong Province, China (Su et al., 2016). Subsequently, this T. urticae population was maintained in the laboratory on detached bean leaves (Phaseolus vulgaris L. cv. Bifeng). A subset of this strain has been continuously reared on tomato (Solanum lycopersicum Miller, cv. Zhongza 9) for more than one year at 26 ± 2°C, with a 60%–80% RH and a photoperiod of L16:D8, and was used in our experiments.
Method details
Gut dissection and protein extraction
Gut tissues of B. tabaci MED adults (Figure S5D) were carefully dissected in ice-cold phosphate-buffered saline (PBS) buffer (pH 7.4) (Sigma-Aldrich). For qPCR analyses, gut tissues and the rest of the body (non-gut body) from about 1000 adults were collected in triplicate and stored in TRIzol reagent (Invitrogen) at −80°C until further use. For malonyltransferase activity measurements, gut tissues were dissected from approximately 10000 adults in PBS buffer containing a protease inhibitor cocktail (Complete tablets EDTA-free, EASYpack, Roche). The obtained gut tissues were pooled, homogenized and ultrasonically disrupted on ice for 6 min. After centrifugation at 12000 × g at 4°C for 10 min, the soluble gut proteins in the supernatant were collected and quantified using the NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific), flash frozen and kept at −80°C until further use.
RNA isolation and cDNA synthesis
Total RNA was extracted from B. tabaci MED samples using TRIzol reagent (Invitrogen) according to the manufacturer’s recommendations. Agarose gel electrophoresis was used to determine the integrity of the RNA, and a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific) was used to quantify the RNA. cDNA was synthesized using the PrimeScript II 1st strand cDNA synthesis kit (TaKaRa) and PrimeScript RT kit (containing gDNA Eraser, Perfect Real Time) (TaKaRa) for BtPMaT1 gene cloning and qPCR analysis, respectively. The synthesized cDNA was immediately stored at −20°C until used.
Gene identification and cloning
The BtPMaT1 gene was found in our recently sequenced B. tabaci MED genome (Gene ID: BTA023005.1, http://gigadb.org/dataset/100286) (Xie et al., 2017) and annotated as a phenolic glucoside malonyltransferase. For the cloning of BtPMaT1, the putative CDS of BtPMaT1 was manually corrected using our previous completed transcriptome data of B. tabaci MED (Tian et al., 2017). Specific primers used for gene cloning (Table S1) were designed using Primer Premier 5.0, and the PCR amplicon from the B. tabaci MED was cloned into the pEASY-T1 (TransGen) vector and transformed into Escherichia coli Trans1-T1 competent cells (TransGen) for sequencing. The full-length cDNA sequence of BtPMaT1 was deposited in the GenBank database (accession number MN756010).
Phylogenetic analysis
The BtPMaT1 protein sequence was used as a query in a tBLASTn and BLASTp search (with “Expect threshold” set at 1E-15) against the B. tabaci MED (http://gigadb.org/dataset/100286) and MEAM1 (http://www.whiteflygenomics.org/cgi-bin/bta/index.cgi) genomes and their predicted protein datasets, respectively (Chen et al., 2016; Xie et al., 2017). BtPMaT1 and BTA005164.2 (BtPMaT2) were subsequently used in a BLASTp and tBLASTn search (with “Expect threshold” set at 1E-15) against the NCBI-non-redundant protein and nucleotide database (version of December 2020), with the “Organism” tab set to “Arthropoda.” Next, BtPMaT1 and BtPMaT2 were used as a query in an unrestricted BLASTp search (with “Expect threshold” set at 1E-15) against the NCBI non-redundant protein database. The top ten hits, together with a random selection of 9-10 hits with a gradually increasing E-value (E-value interval of E-10), were downloaded for each BLASTp search. BtPMaT1 and BtPMaT2 were also used in a BLASTp search against the NCBI non-redundant protein database, with Viridiplantae and B. tabaci excluded in the “Organism” tab and the top ten BLASTp hits with a sequence length of more than 400 amino acids (aa) were retrieved. Last, BtPMaT1 and BtPMaT2 were used in a BLASTp search against the model plant A. thaliana at the TAIR database (Berardini et al., 2015) and the resulting hits (> 400 aa in length, E-value < E-15) were merged with those collected as described above, a set of previously characterized plant malonyltransferases [GmIMaT3, GmMaT4, MtMaT4 Ss5MaT1, Vh3MaT1, Lp3MaT1 and NtMaT1 (Bontpart et al., 2015)] and two basal members of the BAHD superfamily of acyltransferases [A. thaliana CER2, and Zea mays Glossy2 (Tuominen et al., 2011; Yu et al., 2009)]. This set of sequences was subsequently filtered for highly similar protein sequences using CD-HIT with the “-c 0.95” option (Li and Godzik, 2006), resulting in a final dataset of 75 sequences. This filtered protein set was then aligned using the online version of MAFFT version 7 with G-INSI-i settings (Katoh and Standley, 2013) (see Data S1) and a maximum likelihood analysis was performed on the CIPRES Science Gateway (Miller et al., 2012) using RaXML-HPC2 version 8.2.12 on XSEDE (Stamatakis, 2014), with 1000 rapid bootstraps and protein model set to “auto” (RaXML options set to “-p 12345 -m PROTGAMMAAUTO -f a -N 1000 -x 12345”). The resulting tree was midpoint rooted and tree layout was further edited using MEGA7 (Kumar et al., 2016).
Bioinformatic analysis
Illumina DNA reads that were generated during sequencing of the genome of B. tabaci MED, B. tabaci MEAM1, B. tabaci Asia II 3 and B. tabaci New World cryptic species and Illumina RNA-seq reads from B. tabaci MED adult polyA-selected RNA (Tian et al., 2017) were mapped as previously described (Van Leeuwen et al., 2012). The read alignments and coverage were visualized by the IGV 2.3 software (http://software.broadinstitute.org/software/igv/). The genome fragments surrounding BtPMaT1 of B. tabaci MED (http://gigadb.org/dataset/100286) and B. tabaci MEAM1 (http://www.whiteflygenomics.org/cgi-bin/bta/index.cgi) were analyzed as described before (Chen et al., 2016; Xie et al., 2017), while any possible synteny between the B. tabaci genomic region that contains BtPMaT1 (and its neighboring serine protease genes) and the T. vaporarorium genome (https://www.ncbi.nlm.nih.gov/assembly/GCA_011764245.1) (Xie et al., 2020) was investigated using a BLAST approach.
gDNA isolation and genome fragment cloning
Genomic DNA (gDNA) was extracted from B. tabaci MED adults using the TIANamp Blood/Tissue/Cell Genomic DNA Extraction Kit DP304 (TIANGEN) following the manufacturer’s recommendations. For target genome fragment cloning, specific primers to amplify the genome region between the BtPMaT1 and neighboring genes were designed using Primer Premier 5.0 (Table S1). Genome fragments obtained by PCR amplification were ligated into the pEASY-T1 vector (TransGen) and subsequently transformed into Trans1-T1 competent cells (TransGen) for sequencing.
qPCR analysis
The gene-specific primers of BtPMaT1 used for the real time-quantitative PCR (qPCR) analysis were designed by Primer Premier 5.0 (Table S1). The 25 μL PCR reactions included 0.5 μL of 50 × ROX Reference Dye (TIANGEN), 0.75 μL of each specific primer, 1 μL of cDNA template, 9.5 μL of ddH2O, and 12.5 μL of 2 × SuperReal PreMix Plus (SYBR Green) (TIANGEN). The qPCR reactions were performed in an ABI 7500 system (Applied Biosystems) with the following protocol: initial denaturation of 94°C for 3 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. The amplification efficiencies were determined by dissociation curve analysis using five 2-fold serial dilutions of B. tabaci cDNA template. Only primers with 90%–110% amplification efficiencies were used for the subsequent studies.Relative quantification was calculated according to the 2-ΔΔCt method (Livak and Schmittgen, 2001), to accurately analyze the expressions of the target gene, the expression data were normalized to the geometric mean of internal genes including elongation factor 1 alpha (EF1-α) (GenBank accession number EE600682) and 60S ribosomal protein L29 (RPL29) (GenBank accession number EE596314) (Li et al., 2013; Vandesompele et al., 2002). Three independent biological replicates and four technical replicates were performed for each whitefly sample.
Metabolite profiling
Each fresh sample (tomato leaves) was immediately frozen with liquid nitrogen and ground into powder. 500 mg tomato leaf powder was extracted with 4 mL methanol/formic acid (HPLC grade, Thermo Fisher Scientific) (9:1 v/v) in 50 mL centrifugal tube at 4°C for 12 h. After centrifugation at 5000 × g for 20 min, 2 mL supernatant was dried up under nitrogen gas flow. The dried extract was dissolved in 1 mL methanol/formic acid (9:1, v/v), then filtered through a 0.22 μm nylon membrane. The extract was stored at −20°C until used. Three biological replicates were performed for each sample.An ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometer (UPLC-QTOF/MS) system (Waters) was used for the identification of widely targeted metabolites in dried tomato leaves samples. The ChemSpider library including the MS and MS/MS spectra was established by the untargeted method based on the total scan ESI (ESI-QTOF-MS/MS). Full time-of-flight (TOF) scans were acquired in the mass range of m/z 100-1500 at positive acquisition mode using the ACQUITY UPLC I-Class/Xevo G2-XS QTOF system (Waters). The acquisition time of mass was 60 min in total, and scan time was 1.5 scan per second. The solvent A and solvent B for UPLC were acetonitrile (HPLC grade, Thermo Fisher Scientific) with 5% formic acid and water with 5% formic acid, respectively. The UPLC was conducted with 60 min run time and 0.15 ml/min flow rate. Solvent A started at 2.5%, ramped to 10% in 5 min, and ramped from 5 to 20 min at 25%. Then solvent A was kept at 25% from 20 min to 25 min, and changed back to 2.5% from 25 min to 60 min. Column temperature and sample room temperature were set at 25°C and 20°C, respectively. The injection needle was rinsed for 3 s before and after injection. The PDA detector was set to scan the range from 190 to 800 nm, and sampling rate was 20 points per second. The commercial metabolites database Progenesis QI (Waters) was used to analyze the mass data. The phenolic glucosides were manually filtered from the identified compounds. Results are shown in Figure 2 and Table S2.
Phenolic glycoside bioassays
Newly emerged adults (0- to 2-day-old) of B. tabaci MED were fed with diet containing one of the following 11 phenolic glycosides in feeding chambers (Figure 3A): phenyl β-D-glucoside (Yuanye Biotech, ≥ 98%), 4-nitrophenyl β-D-glucoside (Yuanye Biotech, ≥ 98%), salicin (MEC, ≥ 98%), androsin (MREDA, ≥ 98%), verbascoside (MEC, ≥ 98%), 4-methylumbelliferone glucoside (Yuanye Biotech, ≥ 99%), polygalaxanthone III (Solarbio, ≥ 98%), rhaponticin (MREDA, ≥ 98%), kaempferol 7-O-glucoside (Yuanye Biotech, ≥ 98%), kaempferol 3-O-glucoside (Yuanye Biotech, ≥ 98%) or phlorizin (Yuanye Biotech, ≥ 98%). Each phenolic glycoside was dissolved in diet solution containing 30% sucrose and 5% yeast extract (weight/volume) until the target concentration of 10 μM was attained. The feeding chamber consisted of a vertically oriented glass tube (2 cm in diameter × 5 cm long), a black cotton plug and a tube sleeve. The top of the glass tube was sealed with one layer of Parafilm (Alcan Packaging) that was stretched as thinly as possible, and 0.2 mL of a diet solution was added to the outer surface of the Parafilm; the diet solution was then covered with another layer of Parafilm, such that the solution was sandwiched between the two layers of Parafilm at the top of the tube. Approximately 60 B. tabaci MED adults (mixed sexes) were then placed in the tube. Next, a black cotton plug was placed in the open end of the glass tube and the tube was covered with a tube sleeve. Tubes with diet solution without phenolic glycoside were used as controls. Each of the 11 treatments (11 phenolic glycosides) and the control was represented by three replicate tubes. The tubes were placed in an incubator at 25°C and 80% RH with a L14:D10 photoperiod. Mortality of B. tabaci adults was assessed after 96 h. Three independent biological replicates were performed for each treatment.
dsRNA synthesis and RNAi assays
To confirm the role of BtPMaT1 in the detoxification of phenolic glycosides, the expression of BtPMaT1 was knocked down by oral delivery of dsRNA to B. tabaci MED adults. Specific dsRNA primers of BtPMaT1 and EGFP (GenBank accession no: KC896843) containing a T7 promoter on the 5′ end were designed (Table S1) using the SnapDragon tool (https://www.flyrnai.org/cgi-bin/RNAi_find_primers.pl) to avoid potential off-target effects. Furthermore, no specific hits with BtPMaT2, other insect or plant genes were detected by a BLASTn search against the NCBI non-redundant database, using the designed dsRNA fragment as query. An alignment of the BtPMaT1 dsRNA fragment with its corresponding region in BtPMaT2 or with XM_026027746.1, the closest homolog of BtPMaT1 in tomato, also showed low nucleotide identity (45.02% and 43.54%, respectively) and only contained identical nucleotide stretches of maximum 5 to 7 bp. dsRNAs were then in vitro synthesized with the T7 Ribomax Express RNAi System (Promega).RNAi was performed with the above described whitefly feeding setup (Figure 3A), using a diet solution containing dsRNA added between the two layers of Parafilm (200 μL of diet solution with 0.5 μg/μl dsRNA). RNAi efficacy was assessed by qPCR after the B. tabaci adults had fed for 48 h. The adults that had fed for 48 h on diet with dsRNA were then fed a solution containing phenolic glycosides or not for 96 h, and the survival was assessed. Each combination of dsRNA treatment and phenolic glycoside (including the diet solution with dsEGFP as control) was represented by three biological replicates.
VIGS assays
To determine the effect of continuous interference with the BtPMaT1 gene on B. tabaci reproduction and performance, virus-induced gene silencing (VIGS) assays were carried out, and oviposition and whitefly survival were assessed. Virus vectors for VIGS (pTRV1 and pTRV2) (Figure 3I) have previously been described (Liu et al., 2002). The experimental protocol is shown in Figure 3J. A 456-bp fragment of the BtPMaT1 gene was cloned from B. tabaci MED using specific primers (Table S1), and the PCR product was then cloned into XbaI-SacI-cut pTRV2 to construct pTRV2-BtPMaT1. A 435-bp fragment of EGFP gene was cloned using specific primers (Table S1), and the PCR product was then cloned into XbaI-SacI-cut pTRV2 to construct pTRV2-EGFP. The pTRV1, pTRV2-BtPMaT1 and pTRV2-EGFP vectors were transferred into A. tumefaciens GV3101 by electroporation, and the bacteria were selected on LB agar plates containing 100 μg/ml of rifampicin and 50 μg/ml of kanamycin. The A. tumefaciens harboring pTRV1, pTRV2-BtPMaT1 and pTRV2-EGFP were validated by PCR amplification. The A. tumefaciens containing pTRV1 and pTRV2 carrying the target gene were added to 5 mL of liquid LB medium containing 100 μg/ml of rifampicin and 50 μg/ml of kanamycin; the cultures were kept for 18 h at 30°C while shaking them at 200 rpm. A 2 mL volume of each culture was then added to 48 mL of liquid LB medium containing antibiotics as above with 200 μM acetosyringone; the cultures were once again kept for 18 h at 30°C while shaking at 200 rpm. The cultures were then centrifuged at 3000 × g for 10 min, and the pellets containing A. tumefaciens were resuspended in 5 mL of infiltration medium (200 μM acetosyringone, 10 mM MES, and 10 mM MgCl2). The suspension was centrifuged again at 3000 × g for 10 min, and the pellets containing A. tumefaciens were resuspended in infiltration medium to obtain a final OD600 of 0.4. A. tumefaciens containing pTRV1 and pTRV2 with the target gene were mixed at a ratio 1:1. The mixture were infiltrated into the two largest true leaves of tomato plants (Solanum lycopersicum Miller, cv. Zhongza 9) using a 1 mL needleless syringe, tomato plants were left covered overnight. The infiltrated tomato plants were kept in a growth chamber at 27 ± 1°C, 70 ± 10% RH, and a photoperiod of L16:D8. After 20 days, RNA was extracted from the leaves of tomato plants and the cDNA was synthesized. With VIGS-specific primers (Table S1) and a tomato cDNA template, PCR was used to determine whether the VIGS vectors successfully infected the tomato host. The successfully infected tomato plants (PMAT-VIGS tomato and EGFP-VIGS tomato) were used for further study.To assess the effect of VIGS on whitefly performance, a clip cage (3.0 cm diameter and 2.5 cm height) was placed on one leaf of 12 successfully infected PMAT-VIGS tomato plants, and 10 newly emerged (< 24 h) female adults of B. tabaci MED were placed in each cage. Seven days later, the number of living adults and eggs laid on the leaf within the clip cages were counted to assess whitefly performance. Considering that B. tabaci is habitually parthenogenetic, and to exclude the effect of mating on the experiment, only female adults were used in this experiment. In an additional bioassay, 20 newly emerged (< 24 h) adults of B. tabaci MED (with the equal proportion of males and females) were added and after 7 days, the B. tabaci mortality in each clip cage was assessed. In all the assays, twelve EGFP-VIGS tomato plants were used as control.
Heterologous expression and protein purification
Recombinant BtPMaT1 was expressed in Spodoptera frugiperda Sf9 cells (Invitrogen) using the Bac-to-Bac Baculovirus Expression System (Invitrogen). The CDS of BtPMaT1 gene (GenBank accession number MN756010) was subjected to codon optimization and synthesized with a C-terminal 6 × His tag sequence (TsingKe Biotech), and the obtained sequence was further validated with specific primers (Table S1). After validation, it was then cloned into the pFastBac1 vector (Invitrogen) to generate the pFastBac1-BtPMaT1 plasmid with BamHI and HindIII. Recombinant pFastBac1-BtPMaT1 plasmids were transfected to E. coli DH10Bac competent cells (Invitrogen), and the positive recombinant plasmids were then transfected to Sf9 cells using the Cellfectin II Reagent (Invitrogen). The Sf9 cells (1.0 × 106 cells/ml) were cultured in Sf-900 II SFM (Thermo Fisher Scientific) at 27 °C until the viral infection was clear, then P1 viral stock was harvested from the cell culture medium by centrifugation at 500 × g for 5 min and stored at 4°C in the dark. Recombinant protein in the pellets was determined by western blots using an Anti-His (C-term)-HRP Antibody and the SuperSignal West Pico Chemiluminescent Substrate (both Thermo Fisher Scientific). To obtain a suitable titer viral stock, the original P1 viral stock with multiplicity of infection (MOI) of 0.08 was transfected to Sf9 cells to generate a P2 stock. The recombinant protein was then expressed in Sf9 cells by transfection with the P2 titer stock. Three days after infection, cells were harvested and washed three times with PBS buffer (pH 7.4), then resuspended in Buffer A containing 1 mM PMSF and lysed by sonication. After centrifugation at 8000 × g at 4°C for 10 min, the recombinant protein in supernatants was purified by affinity chromatography using a His-Trap HP column (GE Healthcare). The sample was loaded with Buffer A (50 mM Tris–HCl, 200 mM NaCl, pH 8.3) and then eluted with buffer A with an imidazole gradient range from 50 to 250 nM. Protein eluates were added to a HiTrap desalting column (GE Healthcare) with Buffer B (50 mM Tris–HCl, pH 8.3) to remove imidazole, and were then subjected to ultrafiltration with Buffer C (50 mM potassium phosphate, 5 mM 2-mercaptoethanol, pH 8.0) in a 30-kDa cutoff Amicon Ultra-0.5 Device (Millipore).
Metabolic experiments
In an initial BtPMaT1 activity survey, the reaction mixture consisting of Sf9-expressed (10 μg) purified BtPMaT1 protein, 0.2 mM of a phenolic glycosides, and 0.2 mM malonyl-CoA (Sigma-Aldrich, ≥ 90%) in 450 μL of Buffer C. The mixture was made separately for each of the 11 tested phenolic glycosides (see Phenolic glycoside assays) and three additional flavonoid glycosides [quercetin 3-O-β-D-glucoside/isoquercetrin (Meilunbio, ≥ 98%), apigenin-7-O-glucoside/apigetrin (Psaitong, ≥ 98%) and naringenin-7-O-glucoside/prunin (Psaitong, ≥ 98%)]. The reaction mixture without the BtPMaT1 protein was used as the control. The reactions were incubated at 30°C for 1 h and were stopped by adding 450 μL of methanol (HPLC grade, Thermo Fisher Scientific). After centrifugation at 12000 × g at 4°C for 20 min, the reaction supernatants were analyzed using the ACQUITY UPLC I-Class/Xevo G2-XS QTOF system (Waters) equipped with an ACQUITY UPLC BEH C18 column (2.1 mm × 50 mm, 1.7 μm particle size). The column temperature and sample temperature were 30°C and 20°C, respectively. An acetonitrile/water (0.1% formic acid) gradient elution was carried out at a flow rate of 0.2 ml/min as follows: 0-3 min (2%–15% acetonitrile), 3-6 min (15%–40% acetonitrile), 6-9 min (40%–60% acetonitrile), and 9-20 min (60%–98% acetonitrile), with an injection volume of 10 μl. Mass spectrometry (MS) was conducted in the range of 50-900 Da (full-scan MS) in negative ion mode, and the data were analyzed with the Progenesis QI/EZ Info software (Waters).For the determination of the enzymatic activity of the Sf9-expressed recombinant BtPMaT1 protein, 0.1 mM of a phenolic glycoside (either kaempferol 3-O-glucoside, kaempferol 7-O-glucoside, rhaponticin, isoquercetrin, apigetrin or prunin) and 0.2 mM malonyl-CoA were added to the reaction mixture with 5 μg of purified BtPMaT1 protein. The mixture was incubated at 30°C for 30 min, each reaction was replicated twelve times. For the determination of the enzymatic activity of the extracted whitefly gut proteins, 0.01 mM of a phenolic glycoside (either kaempferol 3-O-glucoside, kaempferol 7-O-glucoside, rhaponticin, isoquercetrin, apigetrin or prunin) and 0.2 mM malonyl-CoA were added to the reaction mixture with 50 μg of whitefly gut proteins. The mixture was incubated at 30°C for 1 h, each reaction was replicated five times. The enzymatic activity was measured by MS as described above, with peonidin-3-glucoside (Sigma-Aldrich, ≥ 97%) as an internal standard. One enzymatic unit was defined as nmol product increment per mg of BtPMaT1 protein per min at 30°C.To determine whether BtPMaT1 can detoxify phenolic glycosides, the mortality of B. tabaci adults was measured after they had fed on a diet solution without phenolic glycoside (control), a solution with a representative phenolic glycoside (kaempferol 3-O-glucoside) (Yuanye Biotech, ≥ 98%) or with its corresponding phenolic malonylglucoside [kaempferol 3-O-(6-malonyl-glucoside)] (BOC Sciences, ≥ 95%) for 96 h. The experimental procedure and treatment concentrations were the same as the aforementioned phenolic glycoside bioassays. Three independent biological replicates were performed for each treatment.
Honeydew analysis
To further determine whether BtPMaT1 is responsible for the malonylation of phenolic glucosides in B. tabaci, we performed RNAi, and then honeydew was collected and analyzed by UPLC-QTOF/MS after feeding whiteflies with kaempferol 3-O-glucoside. Kaempferol 3-O-glucoside was dissolved in diet solution (described above) with the final concentration of 10 μM. The honeydew collection device consists of a vertically oriented plastic tube (6 cm in diameter × 1 cm long), and a plastic bottom covered with tinfoil. Two hundred newly emerged whitefly adults fed dsRNA for 48 h on diet solution were transferred into the honeydew collection device, and honeydew was then collected and weighed after adults fed on the phenolic glycoside solution for 48 h. The obtained honeydew was dissolved in deionized water to the final concentration of 0.1 g/ml and isochoric methanol was added. Kaempferol 3-O-glucoside and its malonylation product were analyzed using QTOF/MS and quantified based on their precursor molecular ions in negative ion mode, m/z 447.0780 for kaempferol 3-O-glucoside and m/z 533.0801 for kaempferol 3-O-malonylglucoside, respectively. Each RNAi treatment (dsEGFP or dsBtPMaT1) was biologically replicated three times.Similarly, honeydew of B. tabaci fed on wild-type and transformed tomato plants (see “Transgenic experiments” section) were collected by transferring newly emerged B. tabaci adults into a clip cage (with tinfoil on the bottom), which was placed on one leaf of tomato plants for 48 h. The honeydew was dissolved with deionized water to the final concentration of 0.1 g/ml then isochoric methanol was added. Whole-scale analysis of phenolic glucosides in honeydew was performed using the same method as for detection of BtPMaT1 activity (UPLC-QTOF-MS; Waters ACQUITY UPLC I-Class/Xevo G2-XS QTOF), and the scan was in the range of 50-1200 Da with full-scan in negative ion mode. The Natural Products Application Library integrated in the UNIFI Scientific Information System (Waters) was used to analyze the MS data to identify potential flavonoids. The flavonoid glucosides and malonylated flavonoid glucosides were manually filtered from the identified compounds. The honeydew experiments were conducted for three biological replicates. Raw data were processed using Progenesis QI for peak picking and alignment, one-way ANOVA with Holm-Sidak’s tests and Variable Importance for the Projection (VIP) plot analysis were used to determine significant differences in phenolic glucosides levels (Figure 5; Table S3), with detailed mass features being shown in Table S4.
Transgenic experiments
Transgenic tomato lines were developed by introducing the hairpin RNA expression vector [pCAMBIA-RNAi-BtPMaT1, containing a cauliflower mosaic virus 35S promoter, driving expression in many plant tissues including the phloem (Nilsson et al., 1996)] into tomato (Solanum lycopersicum cv. Moneymaker). The construction of hairpin RNA expression vector (Figure S7A) has been described previously (Xiong et al., 2013). A 444-bp target fragment of BtPMaT1 was cloned from B. tabaci MED using Sense-BtPMaT1 primers (Table S1), and the PCR product was then cloned into XhoI-BglII-cut pRNAi1017 (pRNAi-Sense-BtPMaT1). The anti-sense fragment of BtPMaT1 was cloned from B. tabaci MED using Anti-sense-BtPMaT1 primers (Table S1), the purified product was then cloned into BamHI-SalI-cut pRNAi-Sense-BtPMaT1 (pRNAi-BtPMaT1). The pRNAi-BtPMaT1 vector was digested with PstI and cloned into PstI-cut pCAMBIA2300-35S-OCS (pCAMBIA-RNAi-BtPMaT1). A. tumefaciens LBA4404 based transformation was used to transfer the recombinant pCAMBIA-RNAi-BtPMaT1 plasmid into tomato as described previously (Park et al., 2003). To verify the success of the transformation, gDNA of putative transgenic and non-transgenic tomato leaf was extracted using the Plant Genomic DNA Kit (TIANGEN), and the extracted DNA was subjected to PCR using detection primers (Table S1). Northern blot analyses were performed to test whether dsRNAs was stably expressed in transgenic tomato lines. Total RNA samples were extracted from transgenic or non-transgenic tomato leaves using TRIzol reagent (Invitrogen). Total RNAs (10 μg per lane) were electrophoretically separated by 1.0% formaldehyde-containing agarose gels, then transferred onto a Hybond-N+ nylon membranes (GE Healthcare). For siRNA analysis, cellular RNA samples (approximately 20 μg per lane) were separated by 15% polyacrylamide gels with 7 M urea and 0.3 M sodium acetate. The separated RNA samples were transferred onto Hybond nylon membranes using Trans-Blot SD. Semi-Dry Transfer Cell (Bio-Rad) according to manufacturer’s instructions, subsequently, cross-linking of RNA was achieved by exposure to UV light. Specific DNA probes were obtained from the PCR products (generated by gene-specific primers) labeled with DIG-UTP (Roche). qPCR analyses were carried out to assess the RNAi efficacy on B. tabaci MED adults feeding on transgenic tomato lines, B. tabaci adults feeding on non-transgenic tomato lines were used as control. RNAi efficacy was determined every two days for seven days.To investigate whitefly performance on the transgenic tomato lines, 20 whitefly adults (with an equal proportion of males and females) were released into clip cages attached to one transgenic tomato plant leaf (as described above). Whitefly mortality was assessed every two days for seven days. Six transgenic and six control plants were used for this study, with 2 clip cages per seedling for a total of 12 per treatment. In addition, a simulated field assay was conducted to further evaluate how whiteflies performed on the transgenic tomato lines. Whitefly adults were collected and transferred to transgenic tomato plants (approximately 500 whiteflies per tomato plant) in a cage (60 cm length, 40 cm width and 80 cm height), mortality was assessed after 7 days of feeding. Five transgenic and five control plants were used for this study.To determine whether the transgenic tomato plants had no undesirable effects on non-target arthropods, 10 fourth-instar nymphs of the aphid M. persicae were placed into a clip cages attached to a tomato leaf. After seven days, the number rather than the mortality of M. persicae in each clip cage was recorded for bioassays owing to the reproductive characteristics including parthenogenesis and ovoviviparity of M. persicae. Six transgenic and six control plants were used in this study, with 2 clip cages per plant and, hence, 12 clip cages per treatment. For simulated field assay, fourth-instar M. persicae were carefully transferred onto tomato plants (150 aphids per tomato plant) in a cage, as described above for the whiteflies. Aphid numbers were recorded after 7 days of feeding. Five transgenic and five control tomato plants were used for this assay.In a separate series of experiments, transgenic and non-transgenic tomato plants were also challenged with the spider mite T. urticae. Adult female mites were transferred to fresh transgenic tomato leaves (20 T. urticae mites per leaf) with wet cotton strips wrapped around the petioles. Then, the tomato leaves were placed on moist sponges in Petri dishes, surrounded by cotton strips to prevent T. urticae from escaping. Every two days, the surviving mites were carefully transferred onto a new tomato leaf of the same type and were maintained under the same conditions. The mortality of spider mites was calculated after seven days. For a simulated field assay, adult spider mite females were carefully transferred onto tomato plants (with approximately 200 T. urticae mites per tomato plant) in a cage, mortality was assessed after 7 days of feeding on the plants. In each case five transgenic and five non-transgenic tomato plants were used.
Quantification and statistical analysis
All the data were analyzed using IBM SPSS Statistics (ver. 23.0) software (IBM Corp.). Data are shown as the means ± SEM. Determination of statistical significance was achieved using one-way ANOVA with Holm-Sidak’s tests (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001).